What is the basic unit of matter a

What is the basic unit of matter? a. b. c. d. nucleus cell proton atom

Identify the three particles that make up an atom and their location by drawing a Bohr model of a Beryllium atom.

How are isotopes identified? a. b. c. d. By number of protons By number of electrons By mass number By atomic number

What type of ion forms when an atom loses an electron? a. Anion b. Cation c. Neutral ion d. Negative ion
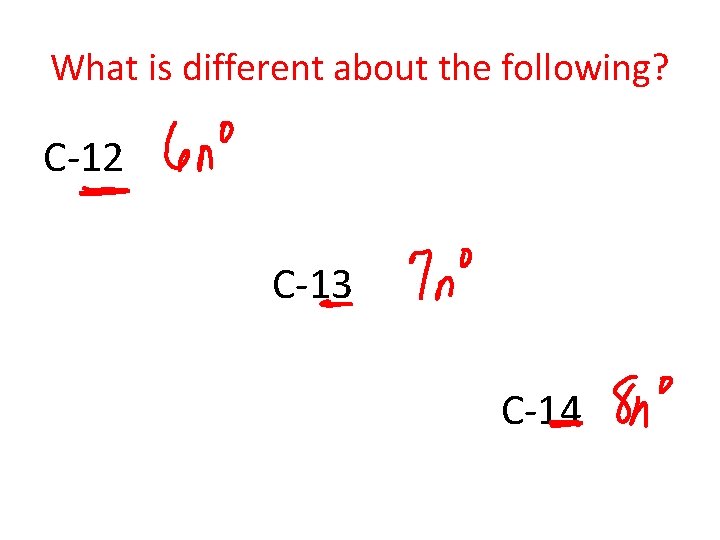
What is different about the following? C-12 C-13 C-14

What are the 2 main types of chemical bonds?
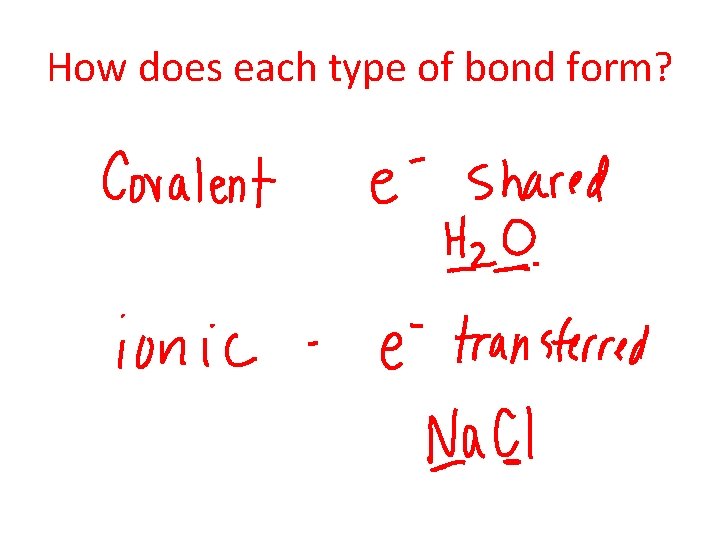
How does each type of bond form?
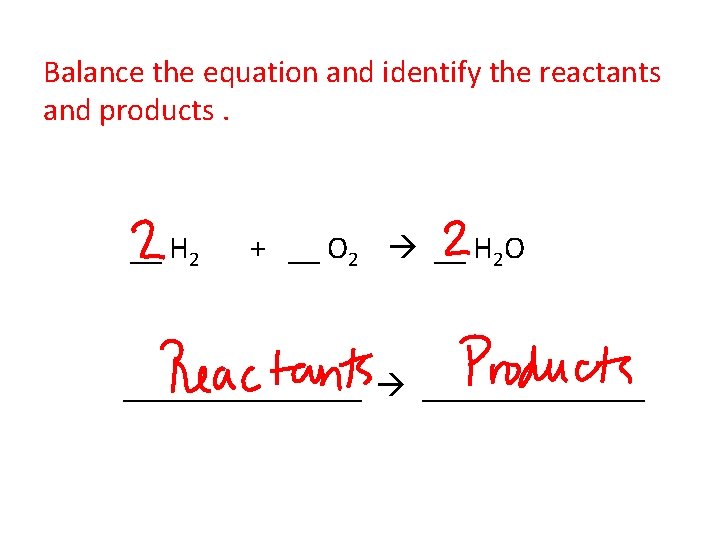
Balance the equation and identify the reactants and products. __ H 2 + __ O 2 __ H 2 O ________

What are the electrons called in the outermost energy level? a. Subshell electrons b. Unstable electrons c. Valence electrons d. Bonding electrons
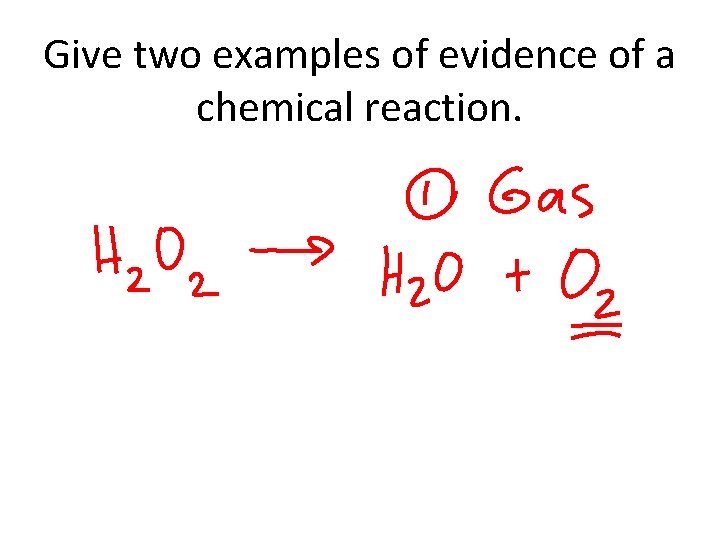
Give two examples of evidence of a chemical reaction.

How do you know a reaction happened in the liver enzyme lab? (What evidence did you observe? )
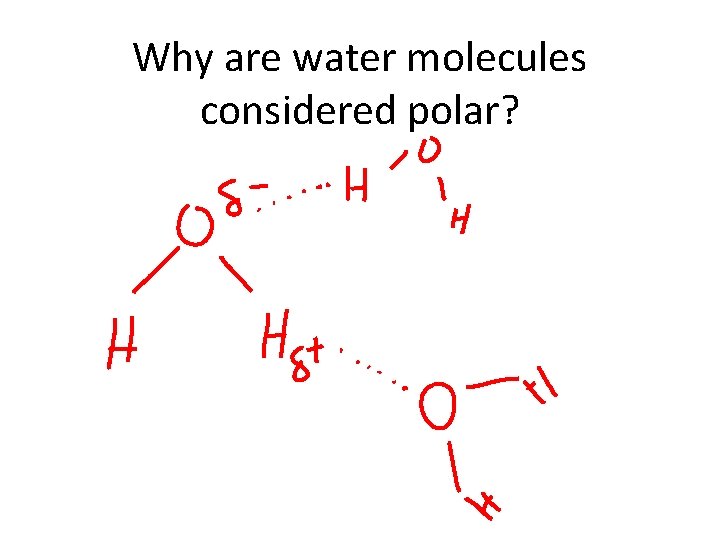
Why are water molecules considered polar?

Name the 4 classes of organic compounds.
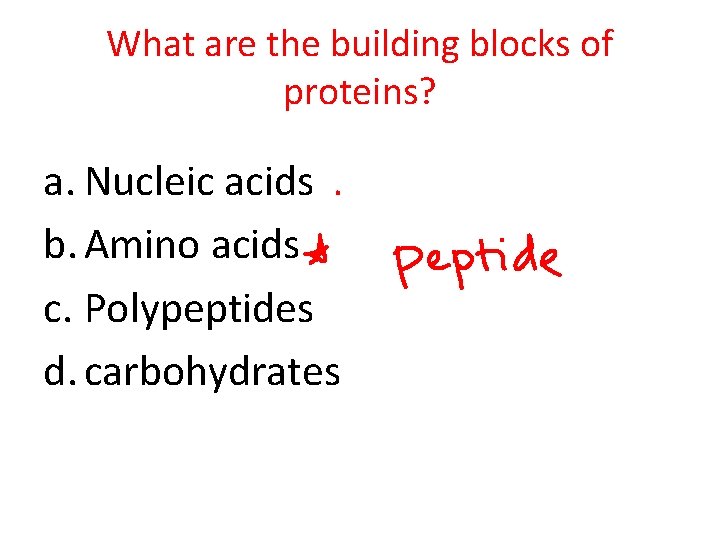
What are the building blocks of proteins? a. Nucleic acids b. Amino acids c. Polypeptides d. carbohydrates
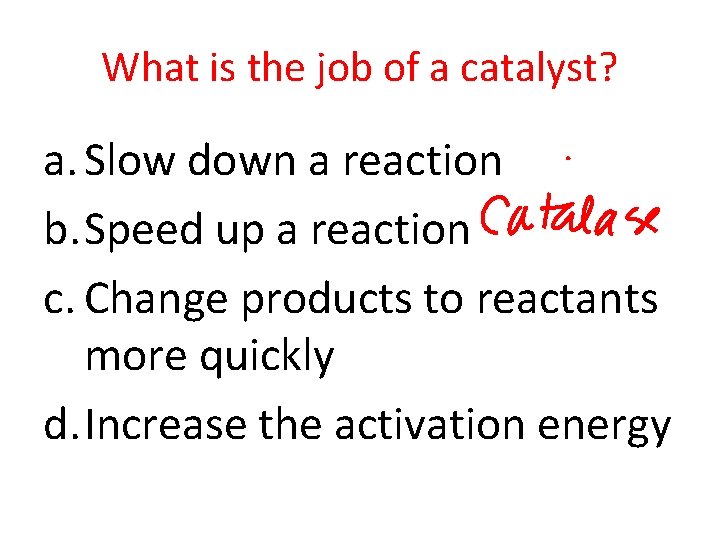
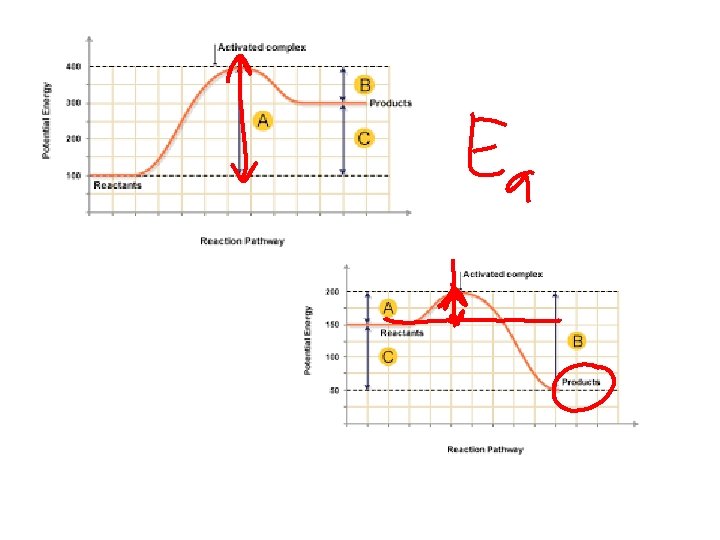
What is the job of a catalyst? a. Slow down a reaction b. Speed up a reaction c. Change products to reactants more quickly d. Increase the activation energy
Name 2 factors that affect enzyme activity.

Test Tuesday over Chapter 2 STUDY!!!!

Be sure to review… • Notes from the chapter • Read the chapter • Milk lab- different organic compounds, +/results, calculations • Liver enzyme lab-how does change in Temp affect enzyme ability/Oxygen production? • Water molecules-cohesion/adhesion • Enzyme activity as a lock and key
- Slides: 19