What is respiration Respiration is the process by
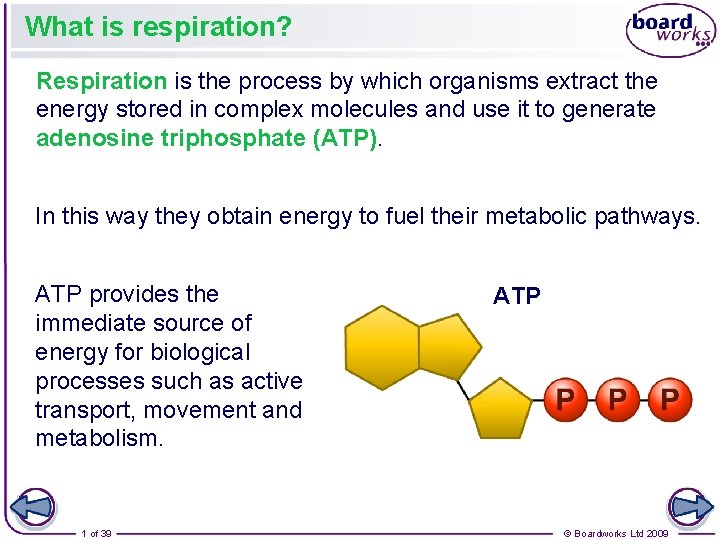
What is respiration? Respiration is the process by which organisms extract the energy stored in complex molecules and use it to generate adenosine triphosphate (ATP). In this way they obtain energy to fuel their metabolic pathways. ATP provides the immediate source of energy for biological processes such as active transport, movement and metabolism. 1 of 39 ATP © Boardworks Ltd 2009
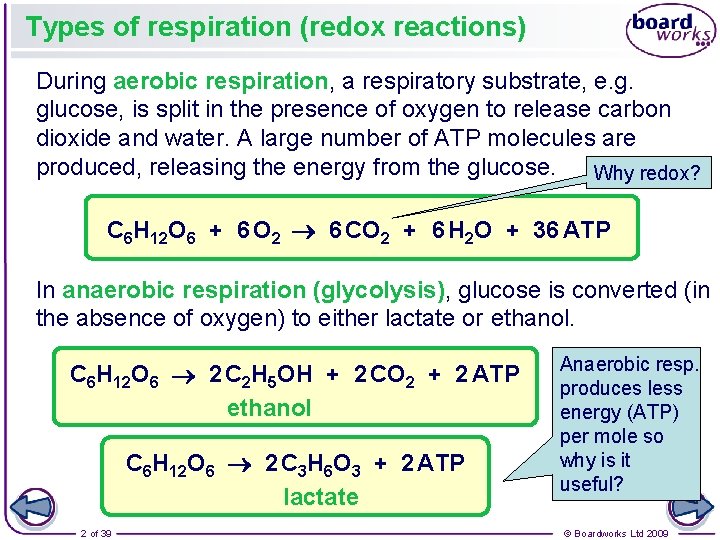
Types of respiration (redox reactions) During aerobic respiration, a respiratory substrate, e. g. glucose, is split in the presence of oxygen to release carbon dioxide and water. A large number of ATP molecules are produced, releasing the energy from the glucose. Why redox? C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + 36 ATP In anaerobic respiration (glycolysis), glucose is converted (in the absence of oxygen) to either lactate or ethanol. C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2 + 2 ATP ethanol C 6 H 12 O 6 2 C 3 H 6 O 3 + 2 ATP lactate 2 of 39 Anaerobic resp. produces less energy (ATP) per mole so why is it useful? © Boardworks Ltd 2009
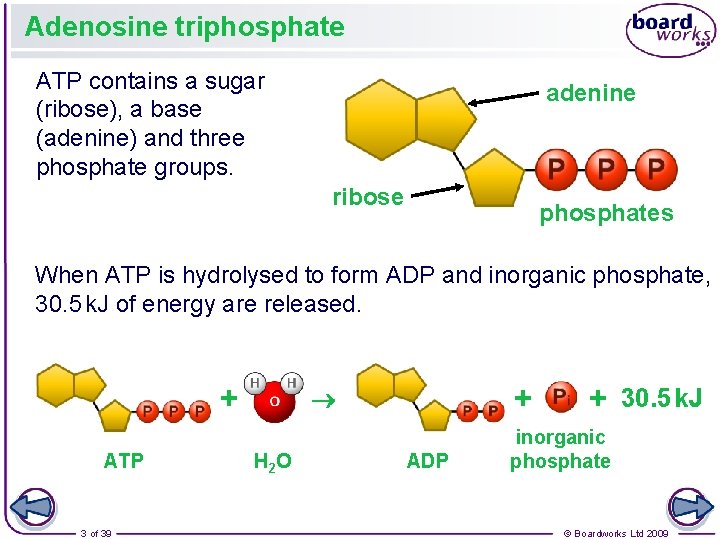
Adenosine triphosphate ATP contains a sugar (ribose), a base (adenine) and three phosphate groups. adenine ribose phosphates When ATP is hydrolysed to form ADP and inorganic phosphate, 30. 5 k. J of energy are released. + ATP 3 of 39 + H 2 O ADP + 30. 5 k. J inorganic phosphate © Boardworks Ltd 2009

Why ATP? Biological systems transfer the energy in glucose to ATP because unlike glucose… glucose ATP l ATP releases its energy instantly in a single reaction. l The hydrolysis of ATP releases a small amount of energy, ideal for fuelling reactions in the body. 4 of 39 © Boardworks Ltd 2009
Phosphorylation of ADP The addition of an inorganic phosphate group (Pi) to a molecule like ADP is called phosphorylation. ADP is phosphorylated during respiration. Two types of phosphorylation occur during respiration: 1. Substrate-level: glycolysis & Krebs cycle A single reaction involving the direct transfer of a phosphate group from a donor molecule to ADP. 2. Oxidative: electron transport chain (cytochrome chain) A series of oxidation reactions that produce sufficient energy to form ATP from ADP and phosphate. 5 of 39 © Boardworks Ltd 2009
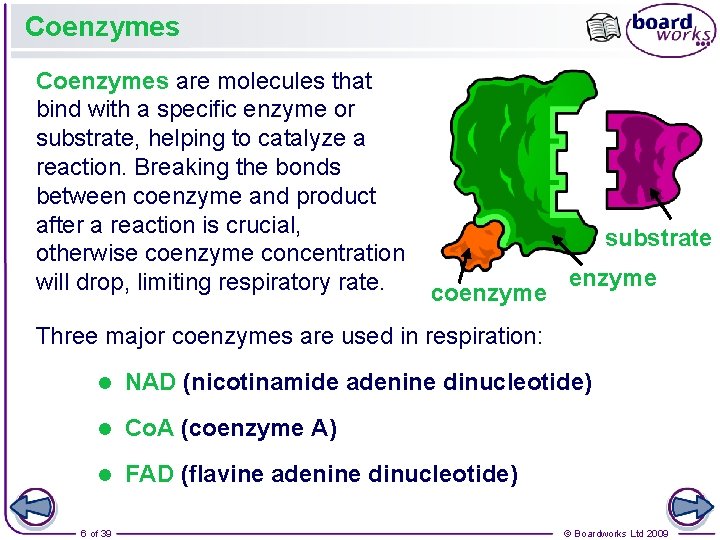
Coenzymes are molecules that bind with a specific enzyme or substrate, helping to catalyze a reaction. Breaking the bonds between coenzyme and product after a reaction is crucial, otherwise coenzyme concentration will drop, limiting respiratory rate. substrate coenzyme Three major coenzymes are used in respiration: l NAD (nicotinamide adenine dinucleotide) l Co. A (coenzyme A) l FAD (flavine adenine dinucleotide) 6 of 39 © Boardworks Ltd 2009
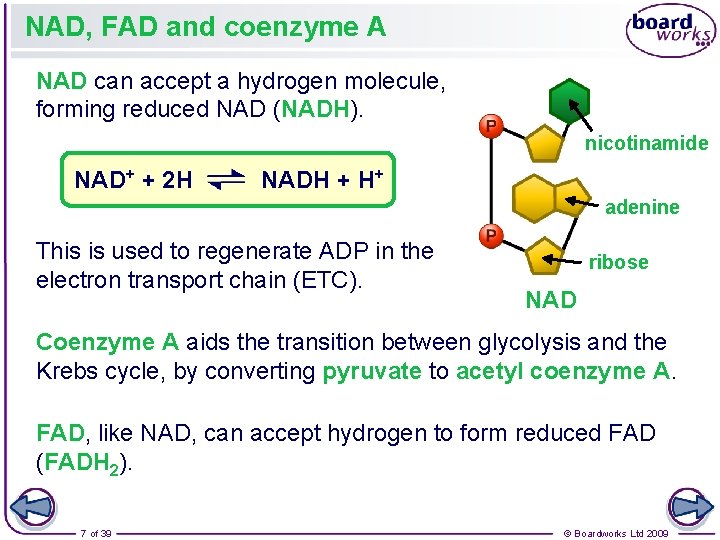
NAD, FAD and coenzyme A NAD can accept a hydrogen molecule, forming reduced NAD (NADH). nicotinamide NAD+ + 2 H NADH + H+ adenine This is used to regenerate ADP in the electron transport chain (ETC). ribose NAD Coenzyme A aids the transition between glycolysis and the Krebs cycle, by converting pyruvate to acetyl coenzyme A. FAD, like NAD, can accept hydrogen to form reduced FAD (FADH 2). 7 of 39 © Boardworks Ltd 2009
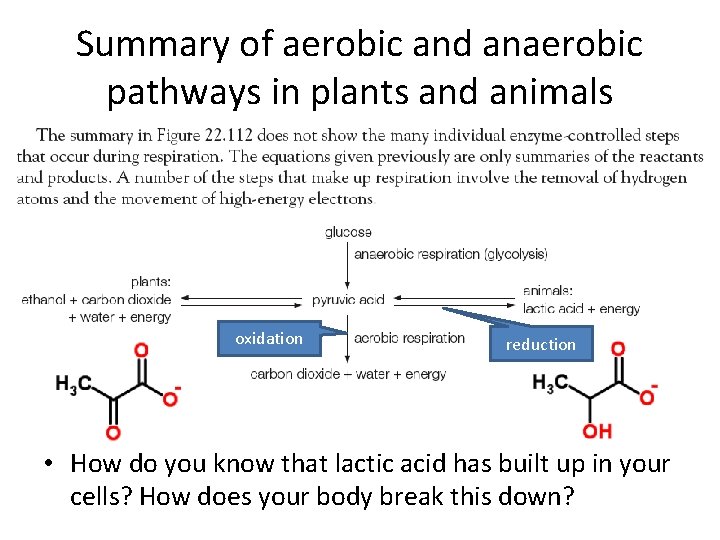
Summary of aerobic and anaerobic pathways in plants and animals oxidation reduction • How do you know that lactic acid has built up in your cells? How does your body break this down?
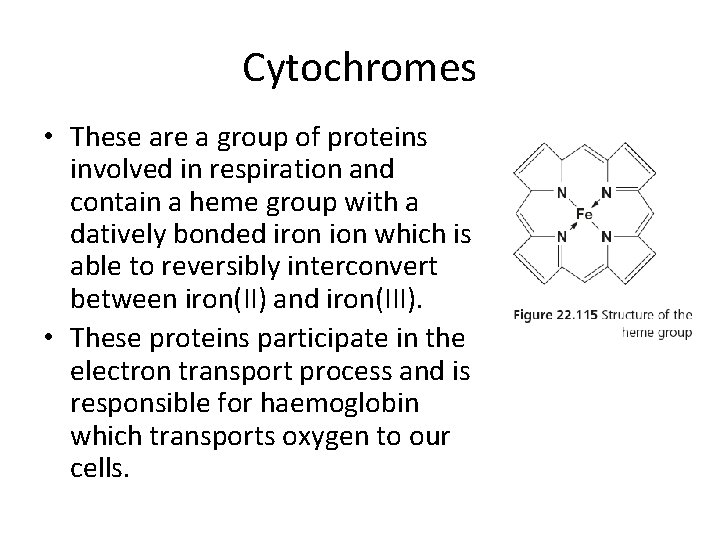
Cytochromes • These are a group of proteins involved in respiration and contain a heme group with a datively bonded iron ion which is able to reversibly interconvert between iron(II) and iron(III). • These proteins participate in the electron transport process and is responsible for haemoglobin which transports oxygen to our cells.
Haemoglobin • The reaction between haemoglobin (Hb) and oxygen can be represented by this simple equilibrium expression: • Hb(aq) + 4 O 2(g) Hb. O 8(aq) • The forward reaction is favoured in the capillaries surrounding the lungs due to the high concentration of oxygen; the reverse reaction is favoured in capillaries near body cells where oxygen concentration are low • Why is CO so dangerous to humans?

Other metal ion/protein reactions • Copper(II) ions are also used in aerobic respiration during which glucose is changed into energy (ATP). • One step involves the transfer of electrons to cytochromes. • As electrons pass from one molecule to the next, copper ions are alternately oxidized and reduced. • Cu 2+ + e– Cu+ • The interconversions of amino acids also require electrons and the oxidation of copper(I) to copper(II) provides these electrons.

Exam question

Solution (5 marks)
- Slides: 13