WHAT IS PHYSICAL SCIENCE Physical Science is the































- Slides: 50

WHAT IS PHYSICAL SCIENCE? � Physical Science is the study of the things around you. � It has to do with matter and energy.

WHAT IS MATTER? � Can you think of examples of matter? � Matter is anything that takes up space
ALL MATTER HAS MASS � What IS mass? � Mass is the amount of material that an object has.

TWO AREAS OF PHYSICAL SCIENCE � Chemistry � Physical Science

PHYSICS � The study of how energy acts with matter

CHEMISTRY � The study of matter and how it changes

TOOLS OF PHYSICAL SCIENTISTS � Scientists answer questions by doing experiments � To do experiments/observations, scientists need tools

Put your name on a piece of paper and number it from 1 through 6 and try to name each of these scientific tools and what you think they do.

METRIC SYSTEM BASICS

METRIC SYSTEM � The metric system is based on a base unit that corresponds to a certain kind of measurement � Length = meter � Volume = liter � Weight (Mass) = gram � Prefixes plus base units make up the metric system � Example: � Centi + meter = Centimeter � Kilo + liter = Kiloliter

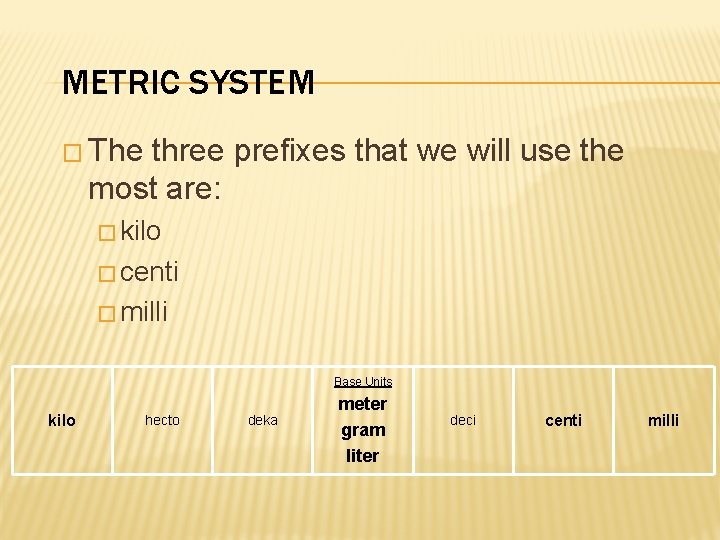
METRIC SYSTEM � The three prefixes that we will use the most are: � kilo � centi � milli Base Units kilo hecto deka meter gram liter deci centi milli

METRIC SYSTEM � So if you needed to measure length you would choose meter as your base unit

METRIC SYSTEM � But what if you need to measure a longer distance, like from your house to school? � Let’s say you live approximately 10 miles from school � 10 miles = 16093 meters � 16093 is a big number, but what if you could add a prefix onto the base unit to make it easier to manage: � 16093 meters = 16. 093 kilometers (or 16. 1 if rounded to 1 decimal place)

METRIC SYSTEM � These prefixes are based on multiples of 10. What does this mean? � From each prefix every “step” is either: � 10 times larger or � 10 times smaller � For example � Centimeters are 10 times larger than millimeters � 1 centimeter = 10 millimeters Base Units kilo hecto deka meter gram liter deci centi milli

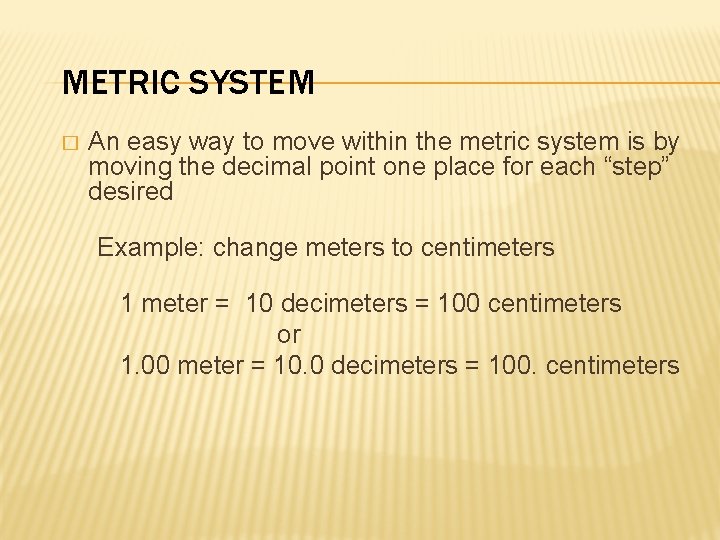
METRIC SYSTEM � An easy way to move within the metric system is by moving the decimal point one place for each “step” desired Example: change meters to centimeters 1 meter = 10 decimeters = 100 centimeters or 1. 00 meter = 10. 0 decimeters = 100. centimeters

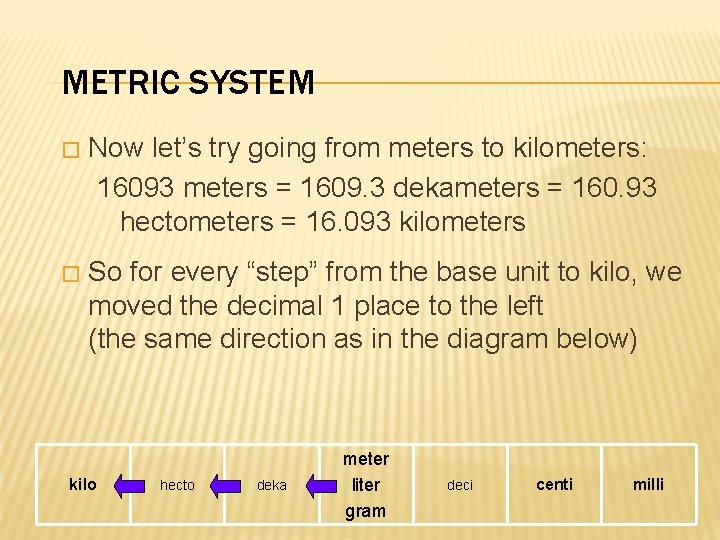
METRIC SYSTEM � Now let’s try going from meters to kilometers: 16093 meters = 1609. 3 dekameters = 160. 93 hectometers = 16. 093 kilometers � So for every “step” from the base unit to kilo, we moved the decimal 1 place to the left (the same direction as in the diagram below) kilo hecto deka meter liter gram deci centi milli

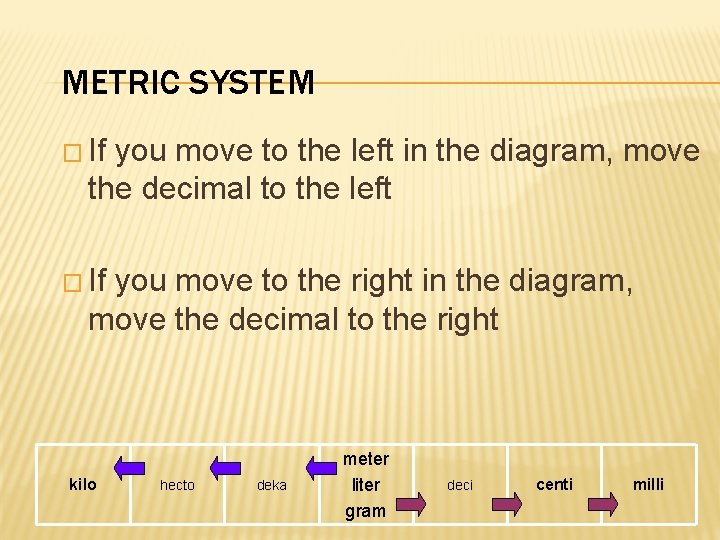
METRIC SYSTEM � If you move to the left in the diagram, move the decimal to the left � If you move to the right in the diagram, move the decimal to the right kilo hecto deka meter liter gram deci centi milli

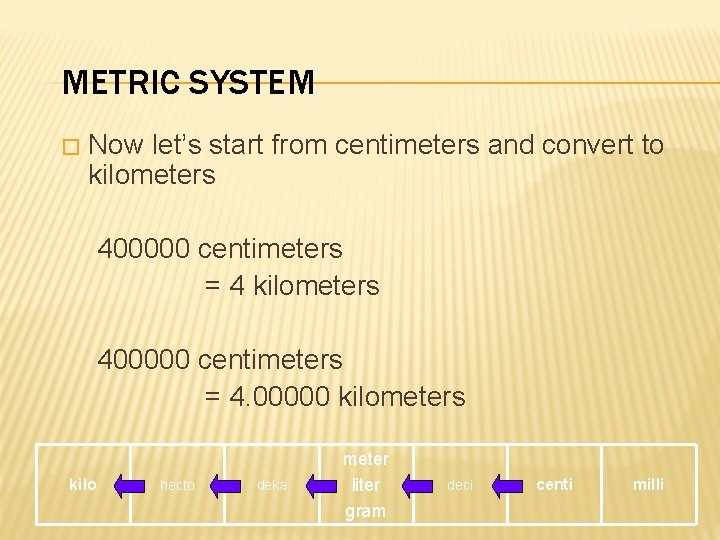
METRIC SYSTEM � Now let’s start from centimeters and convert to kilometers 400000 centimeters = 4. 00000 kilometers kilo hecto deka meter liter gram deci centi milli

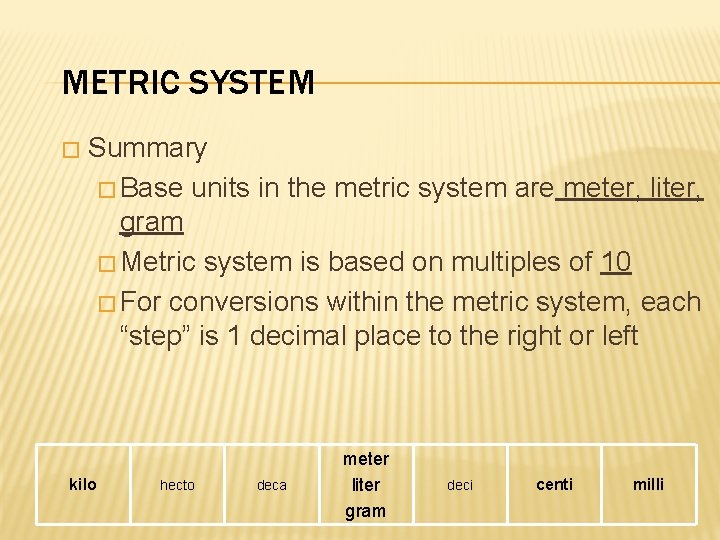
METRIC SYSTEM � Summary � Base units in the metric system are meter, liter, gram � Metric system is based on multiples of 10 � For conversions within the metric system, each “step” is 1 decimal place to the right or left kilo hecto deca meter liter gram deci centi milli

FORMULAS FOR AREA & PERIMETER

PERIMETER � Any shape’s “perimeter” is the outside of the shape…like a fence around a yard. � To calculate the perimeter of any shape, just add up the lengths of all sides 21

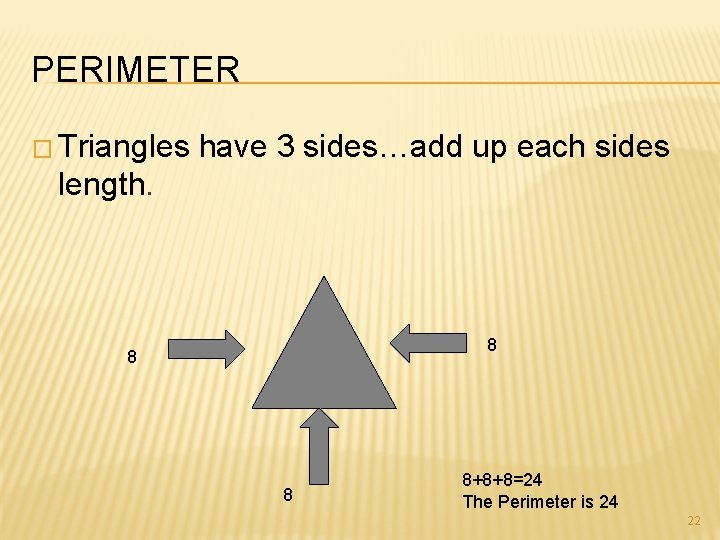
PERIMETER � Triangles have 3 sides…add up each sides length. 8 8+8+8=24 The Perimeter is 24 22

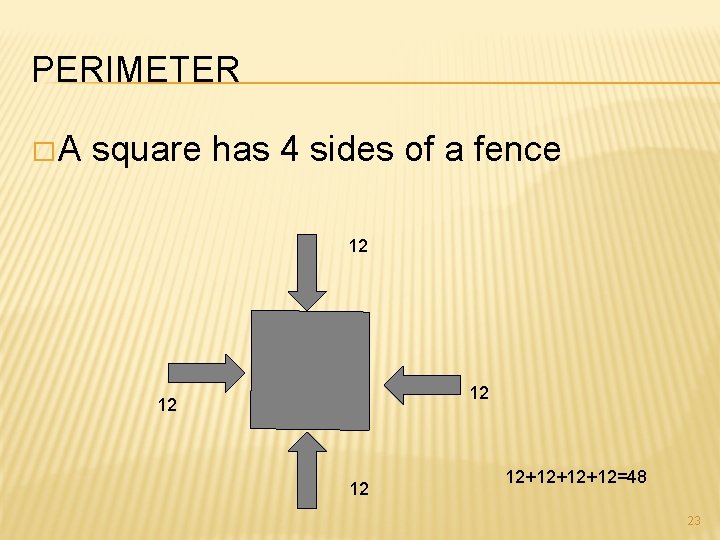
PERIMETER � A square has 4 sides of a fence 12 12 12+12+12+12=48 23

REMEMBER � Squares ALL sides are equal…so if they give you one side, you know ALL the sides � Length=the Largest side � If numbers are left out, they are equal to their opposite side. For example, if you see a number at the bottom of a rectangle then the top of the rectangle is going to be equal to the measurement’s bottom. 24

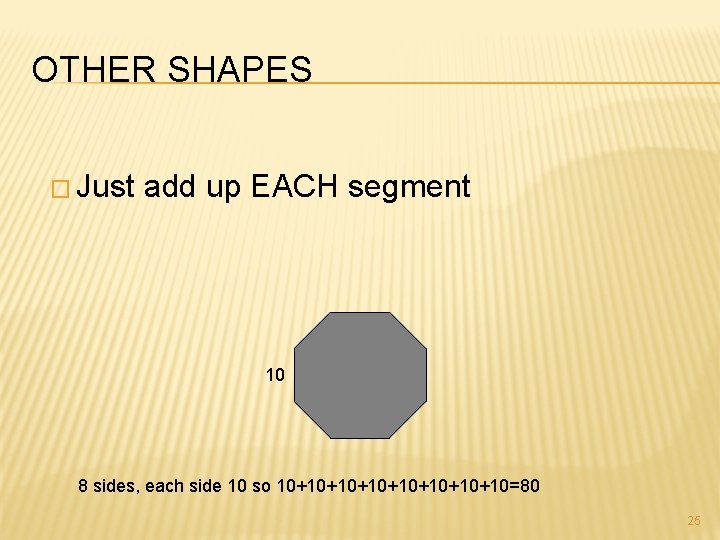
OTHER SHAPES � Just add up EACH segment 10 8 sides, each side 10 so 10+10+10+10+10=80 25

AREA � Area is the ENTIRE INSIDE of a shape � It is always measured in “squares” (cm 2, m 2) 26

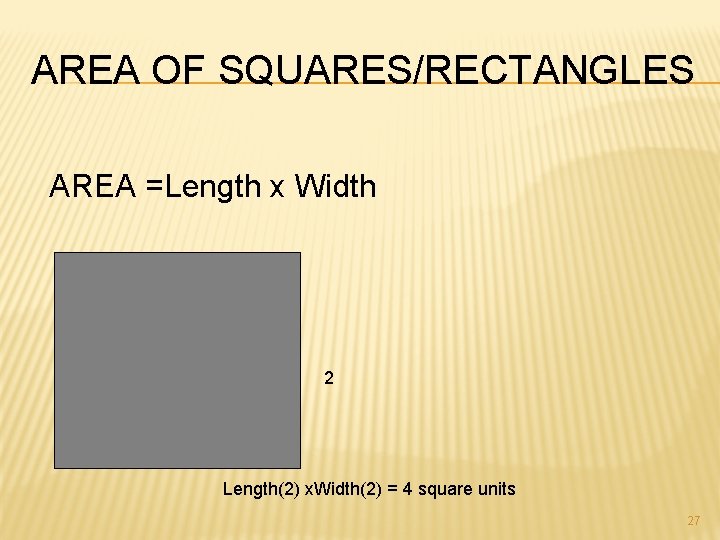
AREA OF SQUARES/RECTANGLES AREA =Length x Width 2 Length(2) x. Width(2) = 4 square units 27

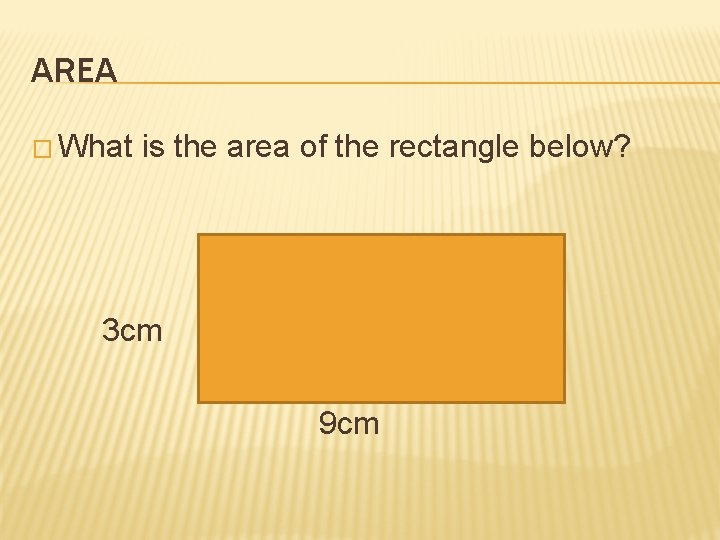
AREA � What is the area of the rectangle below? 3 cm 9 cm

VOLUME

. VOLUME � Volume works with all three dimensions of an object (length, width, and height) and measures the space that an object takes up. � Volume= Length X Width X Height

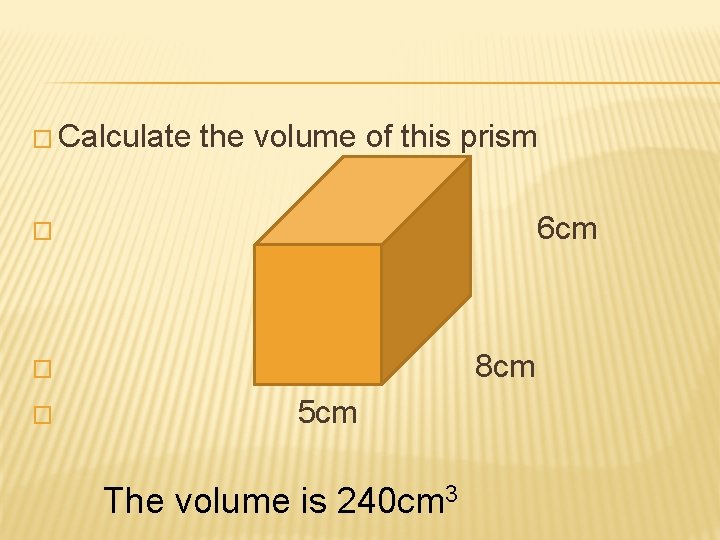
� Calculate the volume of this prism � 6 cm � 8 cm � 5 cm The volume is 240 cm 3

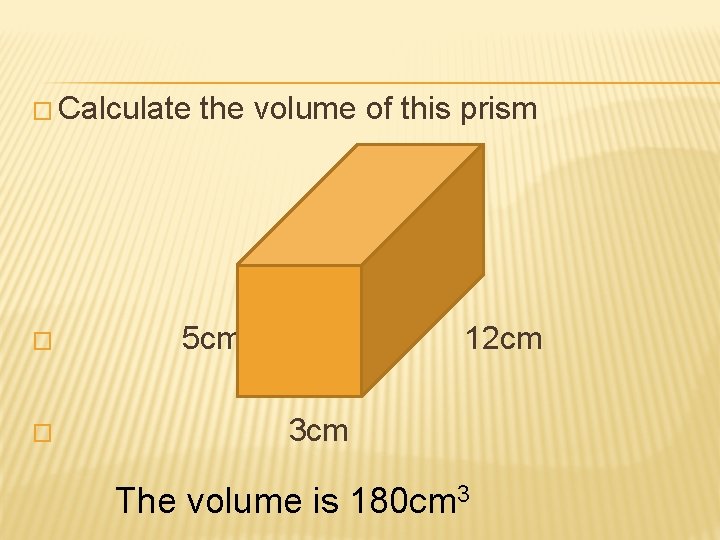
� Calculate the volume of this prism � 5 cm 12 cm � 3 cm The volume is 180 cm 3

Liquid Volume & Volume of Irregular objects

Measuring Volume We will be using graduated cylinders to find the volume of liquids and other objects. Read the measurement based on the bottom of the meniscus or curve. When using a real cylinder, make sure you are eye-level with the level of the water. What is the volume of water in the cylinder? _____m. L What causes the meniscus? A concave meniscus occurs when the molecules of the liquid attract those of the container. The glass attracts the water on the sides. Top Image: http: //www. tea. state. tx. us/student. assessment/resources/online/2006/grade 8/science/images/20 graphicaa. gif Bottom Image: http: //morrisonlabs. com/meniscus. htm

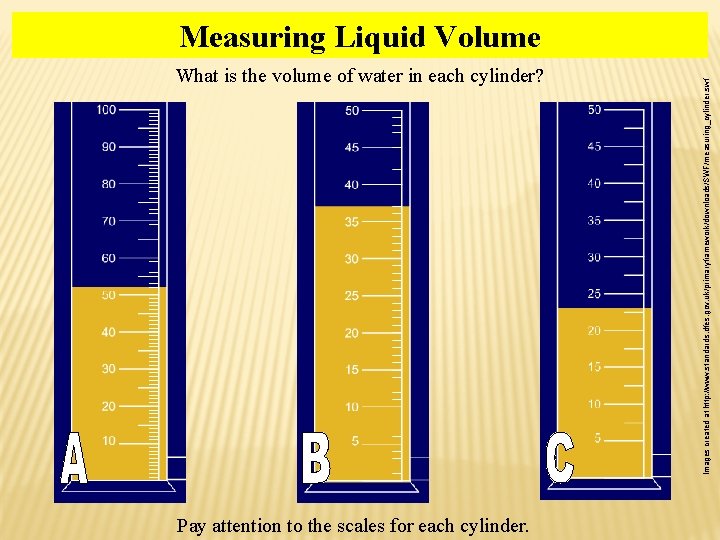
What is the volume of water in each cylinder? Pay attention to the scales for each cylinder. Images created at http: //www. standards. dfes. gov. uk/primaryframework/downloads/SWF/measuring_cylinder. swf Measuring Liquid Volume

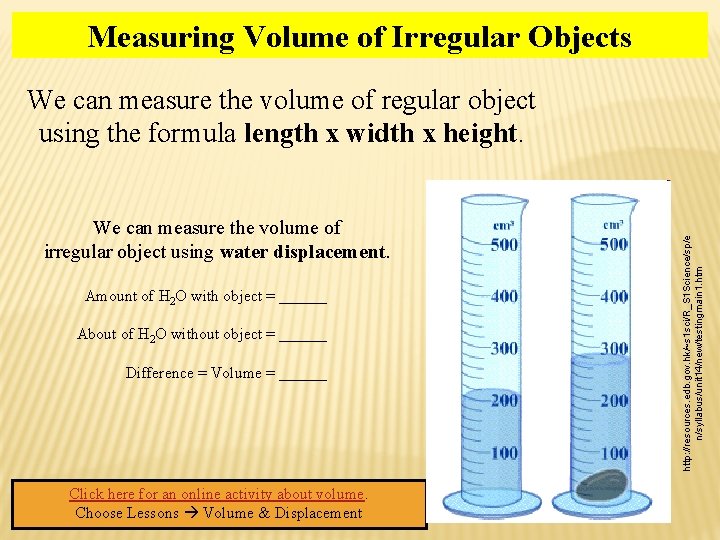
Measuring Volume of Irregular Objects We can measure the volume of irregular object using water displacement. Amount of H 2 O with object = ______ About of H 2 O without object = ______ Difference = Volume = ______ Click here for an online activity about volume. Choose Lessons Volume & Displacement http: //resources. edb. gov. hk/~s 1 sci/R_S 1 Science/sp/e n/syllabus/unit 14/new/testingmain 1. htm We can measure the volume of regular object using the formula length x width x height.

DENSITY

DENSITY � Density is a physical property of matter that describes how closely packed together the atoms of an element or molecules of a compound are. � The more closely packed together they are, the more dense the object. Hence, it can be helpful to know the densities

DENSITY � Density involves mass AND volume of an object! � Mass is the amount of matter contained in an object and is commonly measured in units of grams (g). � Volume is the amount of space taken up by an object � Units used for volume: � cubic centimeters (cm 3) for solids � milliliters (m. L) for liquids � FYI 1 cm 3 = 1 m. L.

DENSITY = MASS/VOLUME � Formula for density = mass divided by volume � Density is a property of matter that is defined as the ratio of an object's mass to its volume. � units for density: � grams per milliliters (g/ml) � grams per cubic centimeter (g/cm 3).

Density of Some Common Substances • Density can be confusing. • For example, many items that we commonly think of as "light" or "heavy" do not have different masses, but they do have different densities. Substance Density (g/cm 3) Air 0. 0013 Feathers 0. 0025 Wood(Oa k) 0. 6 - 0. 9 Ice 0. 92 Water 1. 00 Bricks 1. 84 Aluminu m 2. 70 Steel 7. 80 Silver 10. 50 Gold 19. 30

EXAMPLE � Water’s density = 1. 0 g/m. L: If object’s density is more than that, it will sink in water, if an object’s density is less than that, it will float in water � If an object’s density = water, it will be suspended in water.

LIQUIDS AND GASES � Density applies not only to solids, but liquids and gases � Example: Hot air rises because it’s less dense than the cool air � Example: Oil floats on top of water

SOLVING FOR DENSITY 1. A student determines that a piece of an unknown material has a mass of 5. 854 g and a volume of 7. 57 cm 3. What is the density of the material, rounded to the nearest hundredth? 5. 854 g divided by 7. 57 cm 3 Density = 0. 7733 g/cm 3

DENSITY � D = 0. 77 g/cm 3 � Will this object sink or float in water? � Float—its density is less than 1. 0 g/m. L � Is this a solid or a liquid? � Solid

SOLVE ON YOUR OWN! � � � Mass = 16 g, Volume = 13. 5 m. L, Density = ? Does it sink or float in water? Is it a solid or liquid? Mass = 45 g, Volume = 6. 7 cm 3, Density = ? Does it sink or float in water? Is it a solid or liquid? Mass = 15. 9 g, Volume = 4. 3 m. L, Density = ? Does it sink or float in water? Is it a solid or liquid?

BUOYANCY

BUOYANCY � The buoyant force acts in the direction opposite to the force of gravity, so it makes an object feel lighter • Buoyant Force – upward force exerted by a fluid on a submerged object

BUOYANCY � Archimedes’ principle states that the buoyant force acting on a submerged object is equal to the weight of the fluid the object displaces

FLOATING AND SINKING � If the weight of the object is greater than • the buoyant force – then the object will sink If the weight of the object is less than the buoyant force then the object will float