What is Matter Matter anything that takes up

- Slides: 10


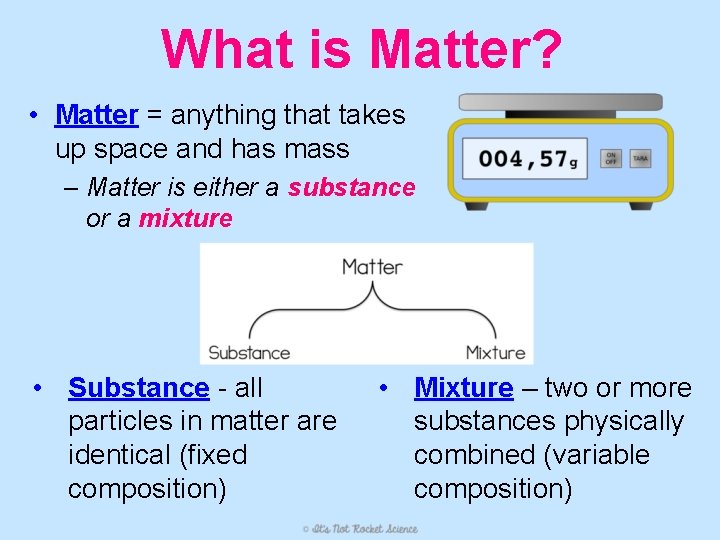
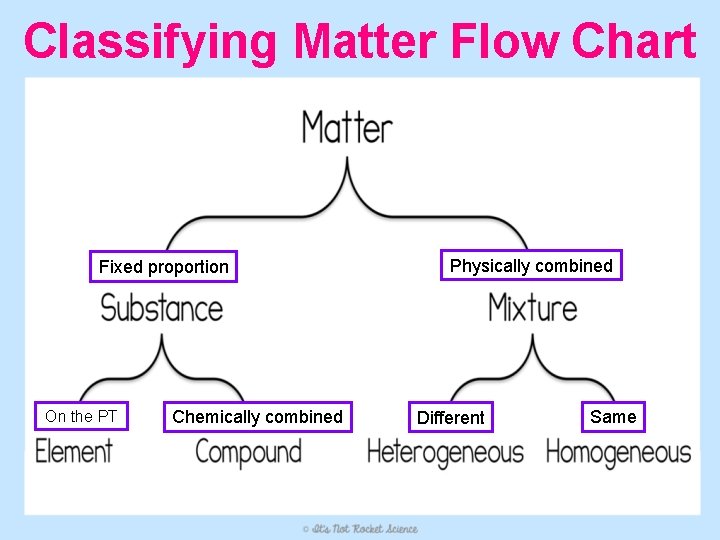
What is Matter? • Matter = anything that takes up space and has mass – Matter is either a substance or a mixture • Substance - all particles in matter are identical (fixed composition) • Mixture – two or more substances physically combined (variable composition)

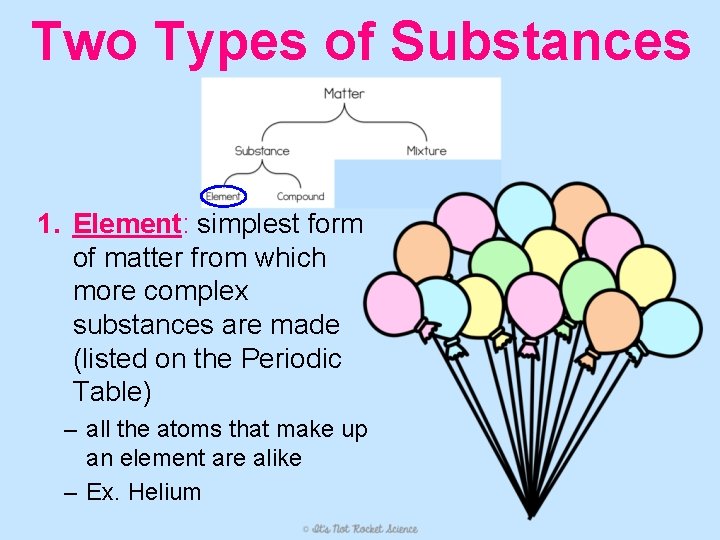
Two Types of Substances 1. Element: simplest form of matter from which more complex substances are made (listed on the Periodic Table) – all the atoms that make up an element are alike – Ex. Helium

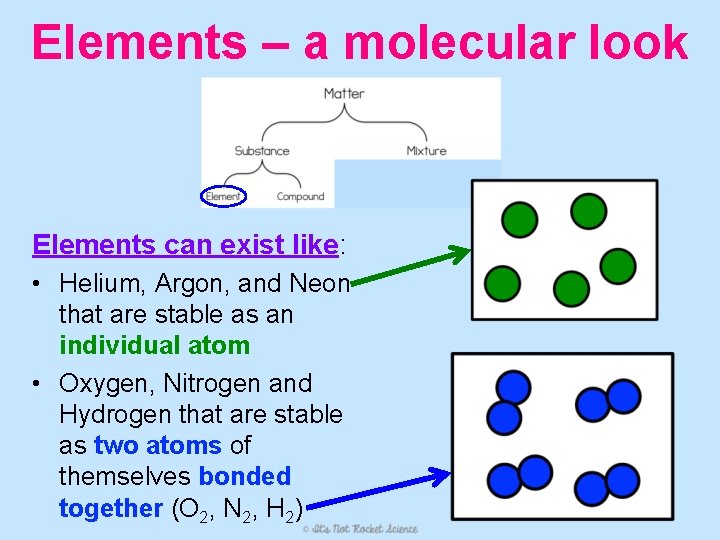
Elements – a molecular look Elements can exist like: • Helium, Argon, and Neon that are stable as an individual atom • Oxygen, Nitrogen and Hydrogen that are stable as two atoms of themselves bonded together (O 2, N 2, H 2)

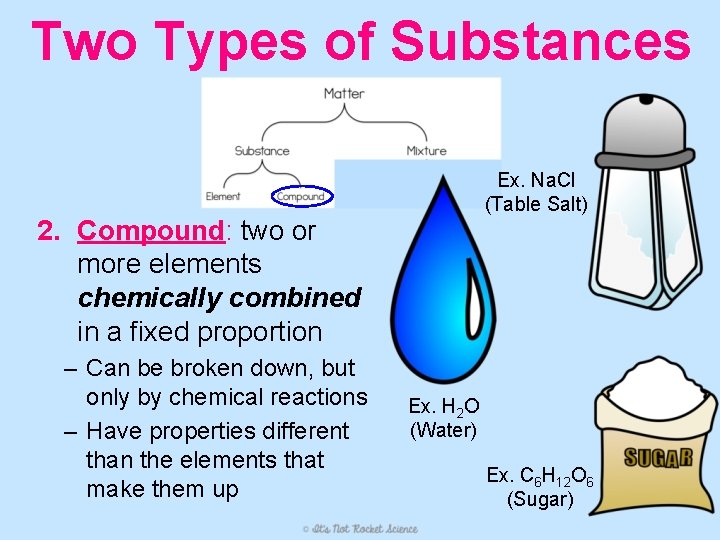
Two Types of Substances Ex. Na. Cl (Table Salt) 2. Compound: two or more elements chemically combined in a fixed proportion – Can be broken down, but only by chemical reactions – Have properties different than the elements that make them up Ex. H 2 O (Water) Ex. C 6 H 12 O 6 (Sugar)

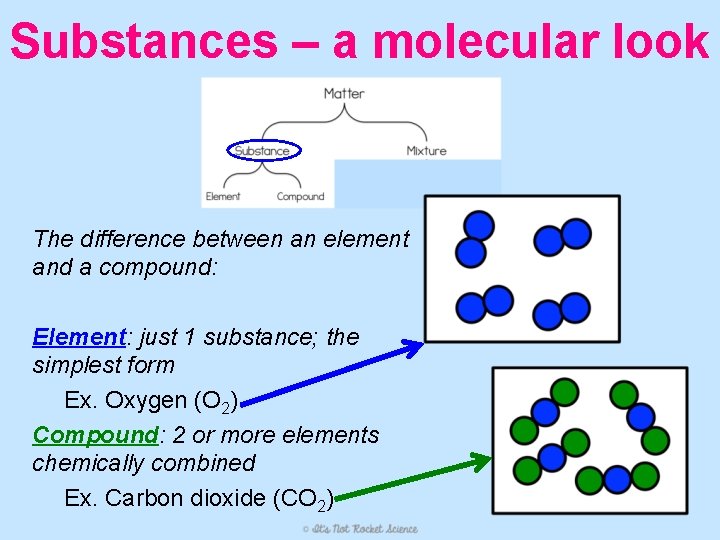
Substances – a molecular look The difference between an element and a compound: Element: just 1 substance; the simplest form Ex. Oxygen (O 2) Compound: 2 or more elements chemically combined Ex. Carbon dioxide (CO 2)

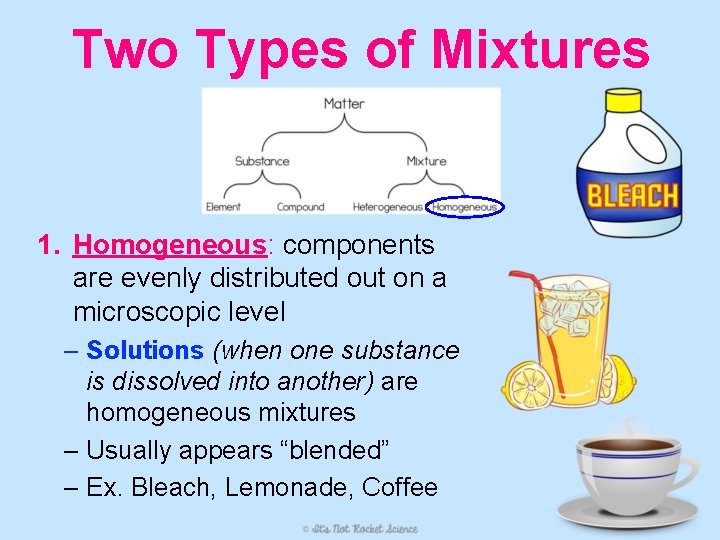
Two Types of Mixtures 1. Homogeneous: components are evenly distributed out on a microscopic level – Solutions (when one substance is dissolved into another) are homogeneous mixtures – Usually appears “blended” – Ex. Bleach, Lemonade, Coffee

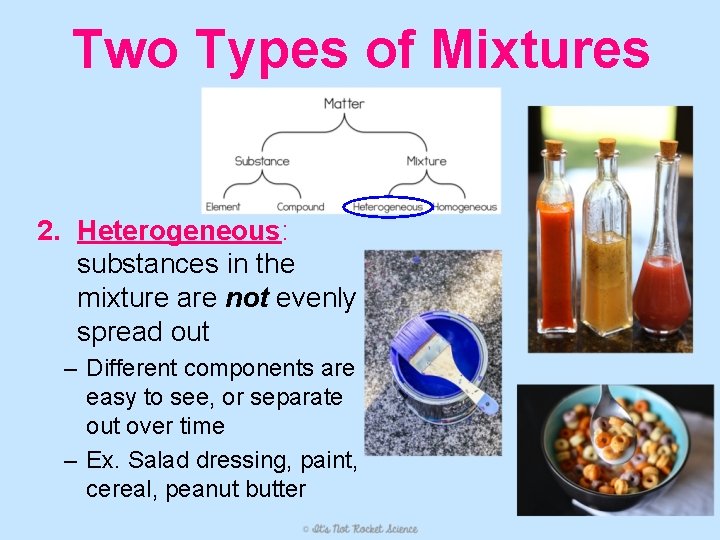
Two Types of Mixtures 2. Heterogeneous: substances in the mixture are not evenly spread out – Different components are easy to see, or separate out over time – Ex. Salad dressing, paint, cereal, peanut butter

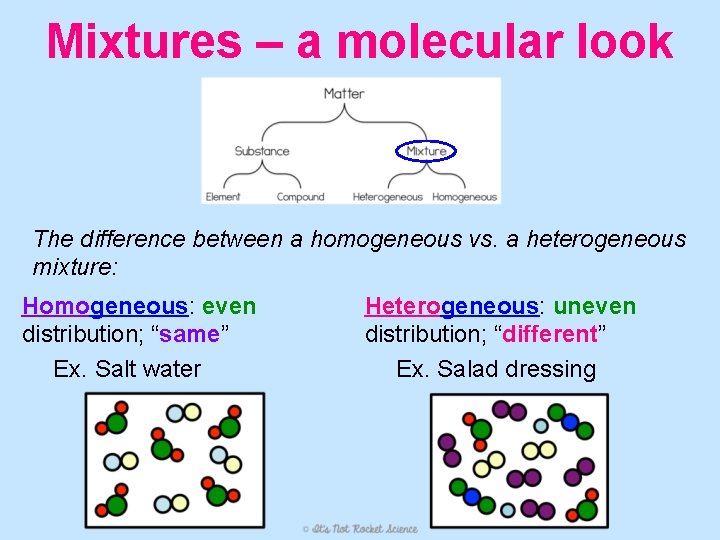
Mixtures – a molecular look The difference between a homogeneous vs. a heterogeneous mixture: Homogeneous: even distribution; “same” Ex. Salt water Heterogeneous: uneven distribution; “different” Ex. Salad dressing

Classifying Matter Flow Chart Fixed proportion On the PT Chemically combined Physically combined Different Same