What is Matter Made Up Of LESSON 1










- Slides: 16

What is Matter Made Up Of? LESSON 1

Matter is Made Up of Atoms �Democritus wondered how many times you could cut something in half and in half until you could not cut it any smaller �He called this the atom! �This was about 2400 years ago! �ATOM – Smallest unit of a pure substance that still has the properties of the substance �It was not until the 1800’s when scientists were finally able to use experiments, not just ideas, to study these things

Atoms are TINY! �How many atoms do you think is in one grain of table salt? � 2, 000, 000! That’s 2 quintillion or 2 billion atoms! �John Dalton used experiments to support the Atomic Theory in the 1800’s, stating that matter is made up of atoms �He also believed that atoms were the smallest particles of matter and cannot be divided

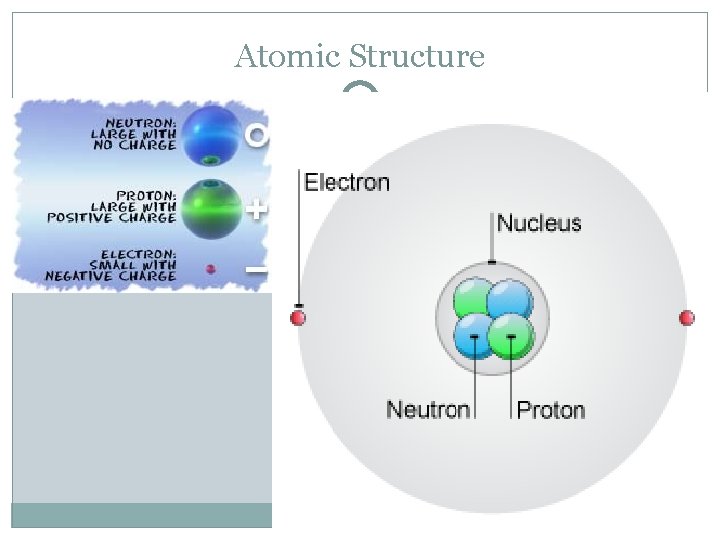
Atomic Structure �J. J. Thomson showed that atoms are made of even smaller particles called subatomic particles �Plum Pudding Model � Does anyone know an example of a subatomic particle? �The atoms is made up many things!: Nucleus Proton Neutron Electron

The Nucleus �The center of an atom which contains protons and neutrons �Ernest Rutherford’s famous experiment in 1911 showed that an atom had a nucleus �Gold Sheet Experiment: �https: //www. youtube. com/watch? v=LMXNESn. Tc 2 c �The nucleus is tiny compared to the size of the atom It is only about 1/10, 000 the diameter of the atom So if the nucleus is a tennis ball, the atom would be the size of about six football fields!

Nucleus �Despite it being a really small portion of the size, it makes up almost the entire mass! �The nucleus is about 99. 9% of the mass of the atom �This is because a proton or neutron has about 2000 X more mass than an electron! �The number of protons contained in the nucleus determines what kind of atom it is

Proton �A proton is a subatomic particle that has a positive charge �Usually found in the nucleus �Every atom’s nucleus contains at least one proton! �The number of protons in an atoms nucleus determines what type of atom it is

Neutron �Subatomic particle that has the same mass as a proton, but does not have electric charge �Neutral charge �Also found in the nucleus

Electrons �Negatively charged subatomic particle �Electrons are in constant motion around the nucleus �Mass is MUCH smaller than proton or neutron �First thought that they were in “orbit” around nucleus �Now we know they are in clouds around the nucleus that define the size of the atom

Atomic Structure

Atomic Number �The number of protons in an atom is called the Atomic Number �For example, the atomic number of Helium is 2, the atomic number of Carbon is 6? �Give me some of your own examples:

Isotopes �All the atoms in an element have the same atomic number because they have the same number of protons! �BUT… Atoms of the same substance may have different masses. �Can anyone think of why this might be? �It is actually because they have a different amount of neutrons! �Atoms that have the same number of protons, but a different number of neutrons are called isotopes!

Atomic Mass Units �Atoms are really small right? !? �How do you think we measure the mass of a single atom? Grams? Pounds? Other guesses? �We instead use atomic mass units or amu �Every proton has the same mass and it is defined as 1 amu �Neutrons have almost the same mass as a proton and so it also has a mass of 1 amu �The atomic mass is the average of all the number of protons and neutrons in all of the isotopes of a substance

AMU and Isotopes �The atomic mass units of hydrogen isotopes are: 1 amu 2 amu 3 amu �An isotope’s names is usually followed by the number of particles in the nucleus �For example, a hydrogen with one proton and no neutrons would be Hydrogen-1

Charges �What is the charge on a proton? �What is the charge on an electron? �I thought opposite charges attract… so why do the electrons stay on the outside of the nucleus and the protons are all next to each other? �The strong nuclear force or atomic force keeps the nucleus together! �An ion is an atom that has an electric charge on it! �A neutral atom has no charge on it! �Let’s do some examples!

�http: //ed. ted. com/lessons/just-how-small-is-an- atom