What is matter Chemistry is the study of














- Slides: 21

What is matter? • Chemistry is the study of matter and how matter interacts with each other • Matter is made up of small particles called atoms – An atom is the smallest part of matter/an element that can be recognized as that element • Back to this later…

Chemical and Physical Properties of Matter • Chemical properties are properties that describe how matter interacts with each other • Physical properties describes the material which composes the matter

Physical Properties • Density- how much matter is packed into a given volume • Malleability- the ability to be pound into a sheet • Ductility- the ability to be drawn into a wire • Conductivity- the ability to conduct an electrical charge • Shape, size, color

Physical Changes • A Physical change does not change the identity of the matter – Examples include: • Change of state of matter (melting, freezing, vaporization) • Cutting a block of wood • Adding dye to water

Chemical Properties • • Reactivity Combustibility p. H Electromotive force

Chemical Changes • A chemical change forms a completely new product – The identity of the matter completely changes – Examples • Rusting • Burning • Salt formation from the neutralization of and acid and or base

Pure Substances • Pure substance – cannot be separated into 2 or more substances by physical or mechanical means – is homogeneous, i. e. , has uniform composition throughout the whole sample – its properties are constant throughout the whole sample – its properties do not depend on how it is prepared or purified – has constant chemical composition

Mixtures • A mixture: – can be separated into 2 or more substances by physical or mechanical means – displays the properties of the pure substances making it up – its composition can be varied by changing the proportion of pure substances making it up – heterogeneous substances, ones with nonuniform composition throughout the sample, are always mixtures

Elements • Classifying Elements – Chemical symbols- are abbreviations for the names of the elements • The first letter of a chemical symbol is always a capital letter • The second (and third) letter (s) are always lower case • A capital letter always indicates a new element • CO= Carbon and Oxygen – Carbon monoxide, a gas • Co= Cobalt, a metal

Compounds • When atoms join together with other atoms to form units they are called molecules • The process of combining different types of atoms is called a chemical change or chemical reaction • Compounds- are the products of chemical changes – A combination of 2 or more different atoms types of elements

Common Compounds • H 2 O- water • Na. Cl- sodium chloride- table salt

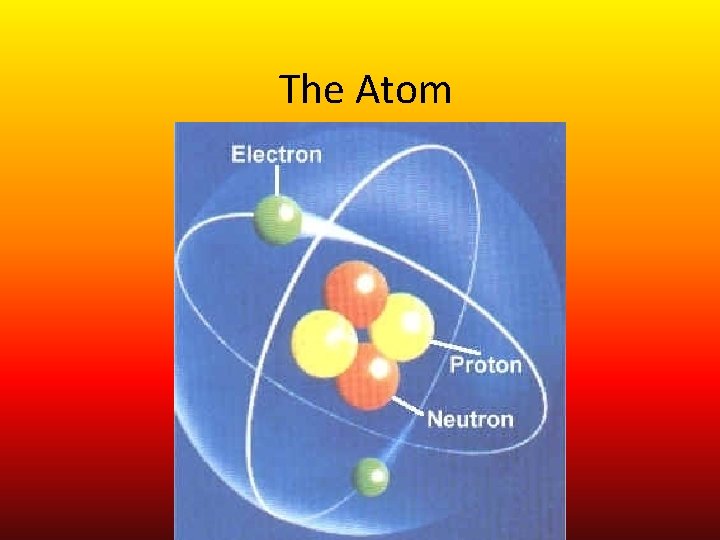
The Atom

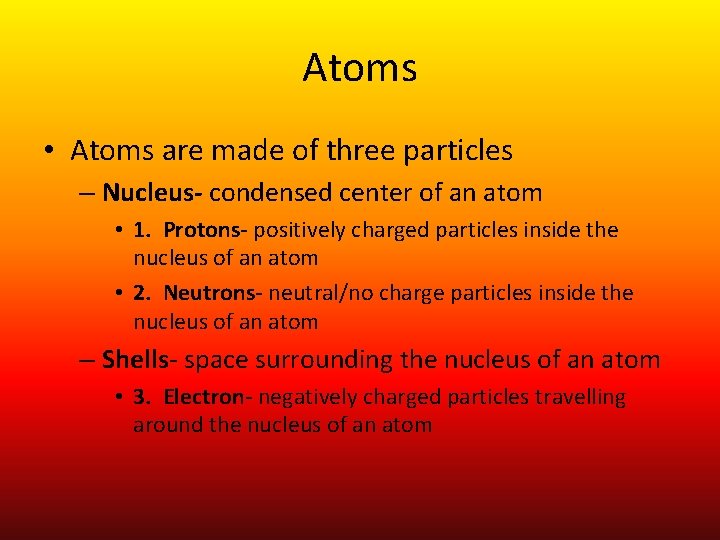
Atoms • Atoms are made of three particles – Nucleus- condensed center of an atom • 1. Protons- positively charged particles inside the nucleus of an atom • 2. Neutrons- neutral/no charge particles inside the nucleus of an atom – Shells- space surrounding the nucleus of an atom • 3. Electron- negatively charged particles travelling around the nucleus of an atom

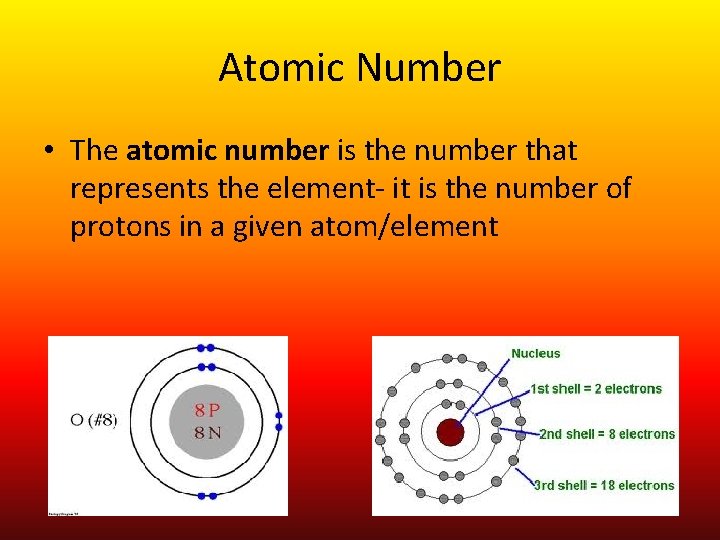
Atomic Number • The atomic number is the number that represents the element- it is the number of protons in a given atom/element

What is energy? • Energy is the ability to do work and these atoms are working all the time.

Energy Forms • Energy comes in many different forms – Potential energy- energy due to position – Kinetic energy in motion – Electrical energy forces that move electrically charged particles – Chemical energy responsible for making and breaking chemical bonds – Nuclear energy cause by the removal or addition of particles in the nucleus of an atom

Conservation of Matter and Energy • Now it is important to note that matter and energy cannot be created nor destroyed only transferred from one form to another – The Law of Conservation of Energy • The 1 st law of thermodynamics

Law of Entropy • Now all of this matter and it energy is constantly changing forms and is constantly moving towards a state of disorder or randomness- entropy

2 nd Law of Thermodynamics • This law states that all natural processes tend toward the highest entropy and minimal useable energy – Entropy or disorder is always increasing

5 States of Matter • Solid- particles are tightly packed, usually in a regular pattern. – particles vibrate (jiggle) but generally do not move from place to place. • Liquid- particles are close together with no regular arrangement. – Particles vibrate, move about, and slide past each other. • Gas- particles are well separated with no regular arrangement. – Particles vibrate and move freely at high speeds.

States of Matter • Plasma- this is an ionized gas. It is gas that has so hot that it acts as a liquid – The particles are packaged similarly to a liquid • Bose Einstein? – It is a super fluid with some odd characteristics – It is super cold, it exists at absolute 0