What is Gene Technology Gene technology is a












- Slides: 71


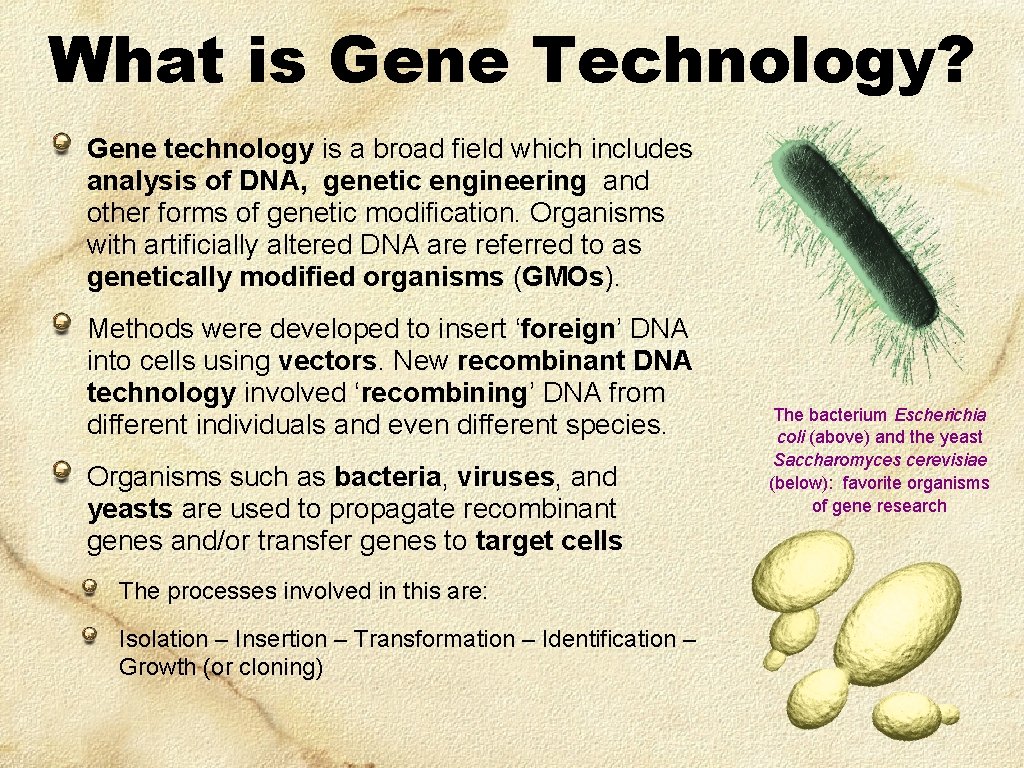
What is Gene Technology? Gene technology is a broad field which includes analysis of DNA, genetic engineering and other forms of genetic modification. Organisms with artificially altered DNA are referred to as genetically modified organisms (GMOs). Methods were developed to insert ‘foreign’ DNA into cells using vectors. New recombinant DNA technology involved ‘recombining’ DNA from different individuals and even different species. Organisms such as bacteria, viruses, and yeasts are used to propagate recombinant genes and/or transfer genes to target cells The processes involved in this are: Isolation – Insertion – Transformation – Identification – Growth (or cloning) The bacterium Escherichia coli (above) and the yeast Saccharomyces cerevisiae (below): favorite organisms of gene research

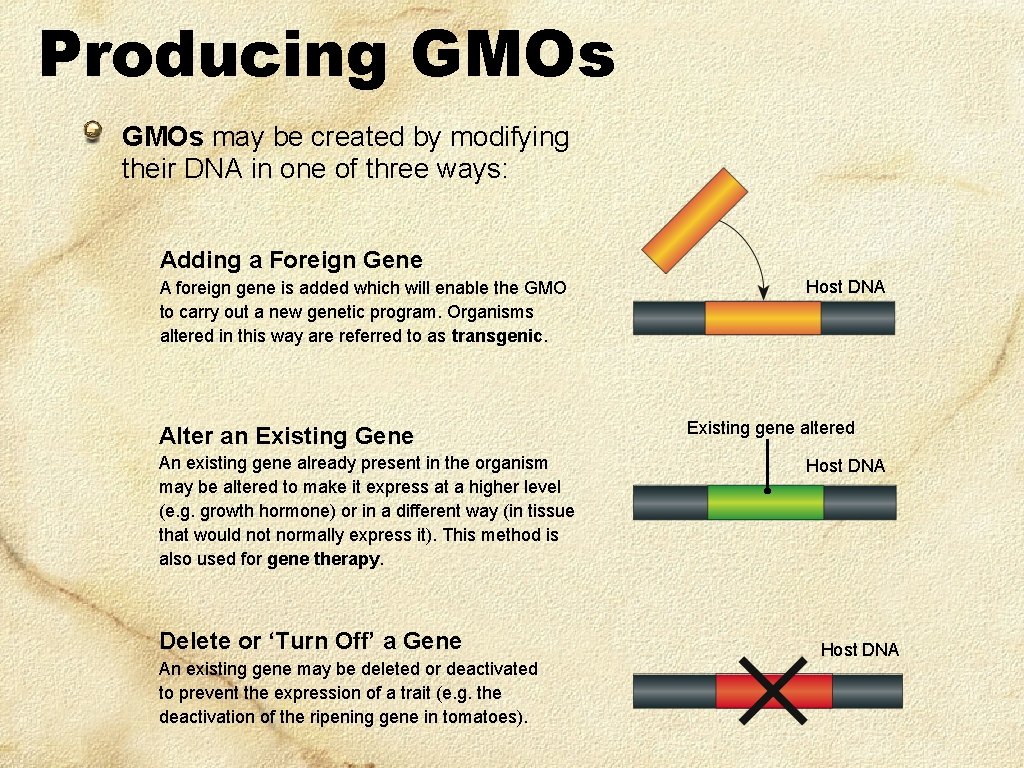
Producing GMOs may be created by modifying their DNA in one of three ways: Adding a Foreign Gene A foreign gene is added which will enable the GMO to carry out a new genetic program. Organisms altered in this way are referred to as transgenic. Alter an Existing Gene An existing gene already present in the organism may be altered to make it express at a higher level (e. g. growth hormone) or in a different way (in tissue that would not normally express it). This method is also used for gene therapy. Delete or ‘Turn Off’ a Gene An existing gene may be deleted or deactivated to prevent the expression of a trait (e. g. the deactivation of the ripening gene in tomatoes). Host DNA Existing gene altered Host DNA

Gene Cloning Gene cloning is a process of making large quantities of a desired piece of DNA once it has been isolated. This can be done in two ways: in vivo – fragments are transferred into another organism, resulting in a recombinant DNA molecule or molecular clone. in vitro – fragments are copied many times over in a laboratory, usually using a technique called the polymerase chain reaction (PCR). Cloning allows for an unlimited number of copies of a gene to be produced for analysis or for production of a protein product.

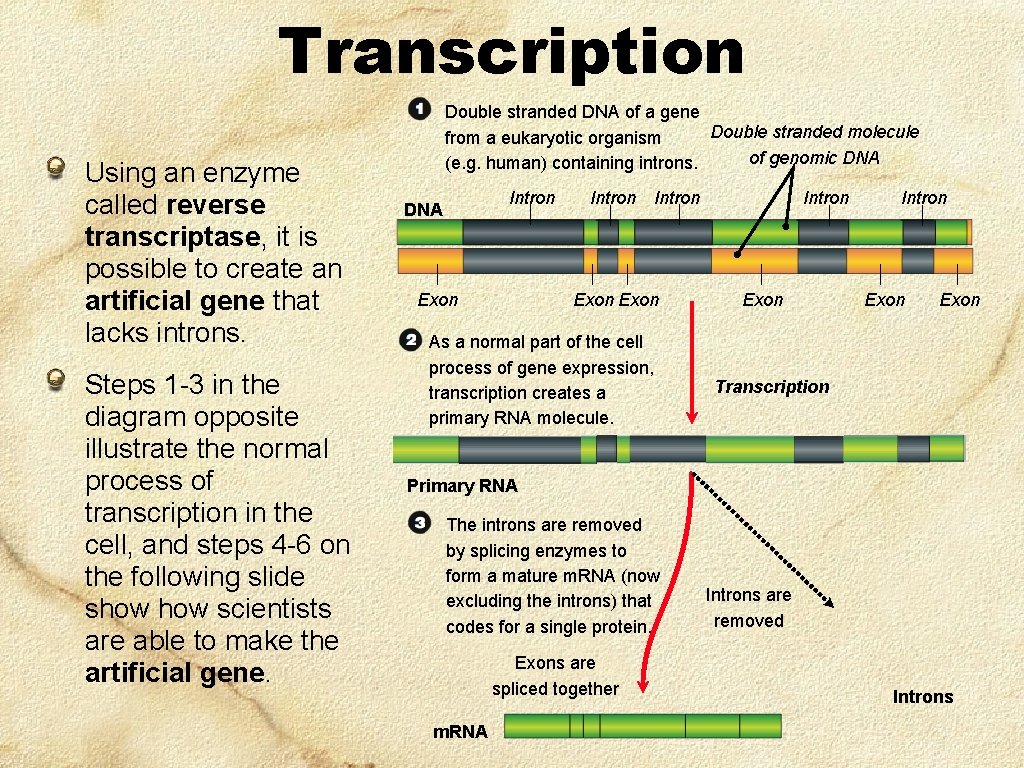
Making an Artificial Gene One problem with DNA from an organism’s cell is that it often contains long introns. Introns can be of enormous length and cause problems when the gene as a whole is inserted into vectors for cloning: Plasmids tend to lose large inserts of foreign DNA. Viruses cannot fit the very long DNA into their protein coats (capsids). To avoid this problem, it is possible to make an artificial gene that lacks introns. This is possible by using an enzyme called reverse transcriptase which is able to reverse the process of transcription by making DNA molecules out of their m. RNA products. The important feature of this process is that m. RNA has already had the introns removed. By using m. RNA as the template to recreate the gene, the DNA will also lack the troublesome intron regions.

Transcription Using an enzyme called reverse transcriptase, it is possible to create an artificial gene that lacks introns. Steps 1 -3 in the diagram opposite illustrate the normal process of transcription in the cell, and steps 4 -6 on the following slide show scientists are able to make the artificial gene. Double stranded DNA of a gene Double stranded molecule from a eukaryotic organism of genomic DNA (e. g. human) containing introns. Intron DNA Exon Intron Exon As a normal part of the cell process of gene expression, transcription creates a primary RNA molecule. Intron Exon Transcription Primary RNA The introns are removed by splicing enzymes to form a mature m. RNA (now excluding the introns) that codes for a single protein. Exons are spliced together m. RNA Introns are removed Introns

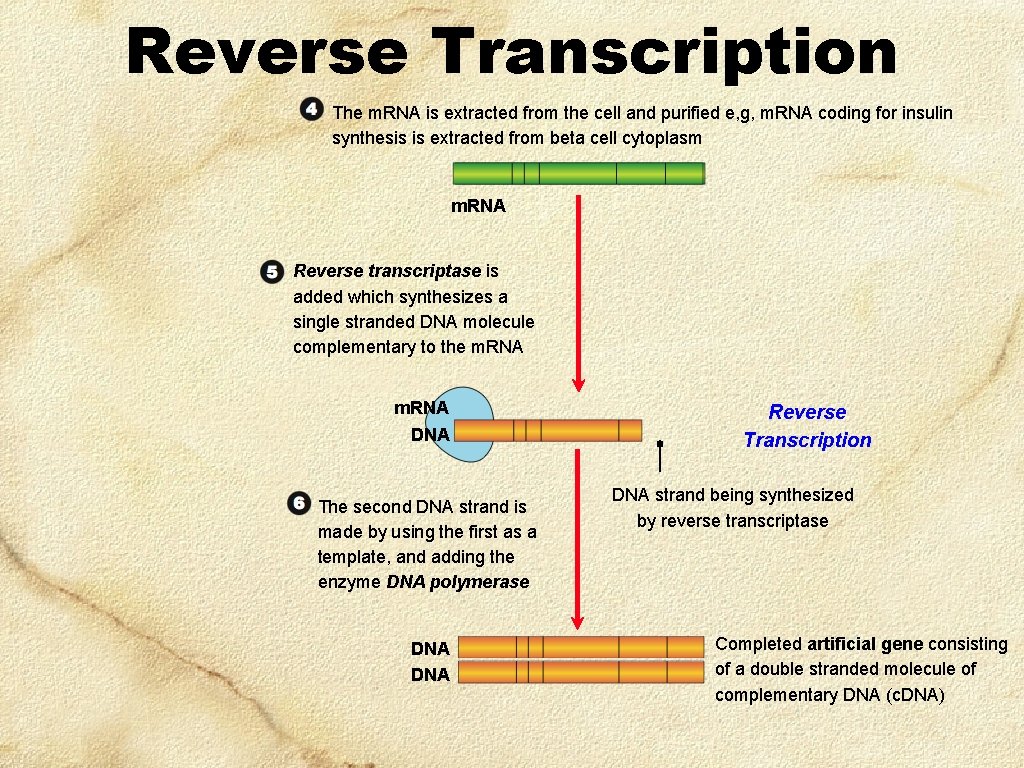
Reverse Transcription The m. RNA is extracted from the cell and purified e, g, m. RNA coding for insulin synthesis is extracted from beta cell cytoplasm m. RNA Reverse transcriptase is added which synthesizes a single stranded DNA molecule complementary to the m. RNA DNA The second DNA strand is made by using the first as a template, and adding the enzyme DNA polymerase DNA Reverse Transcription DNA strand being synthesized by reverse transcriptase Completed artificial gene consisting of a double stranded molecule of complementary DNA (c. DNA)

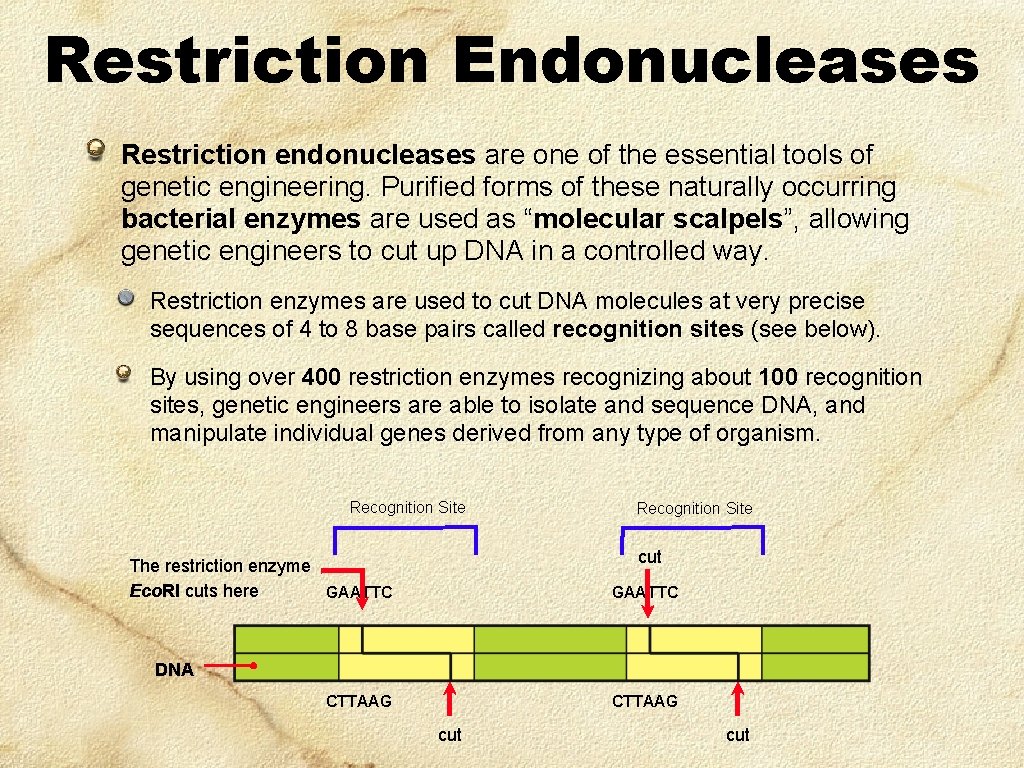
Restriction Endonucleases Restriction endonucleases are one of the essential tools of genetic engineering. Purified forms of these naturally occurring bacterial enzymes are used as “molecular scalpels”, allowing genetic engineers to cut up DNA in a controlled way. Restriction enzymes are used to cut DNA molecules at very precise sequences of 4 to 8 base pairs called recognition sites (see below). By using over 400 restriction enzymes recognizing about 100 recognition sites, genetic engineers are able to isolate and sequence DNA, and manipulate individual genes derived from any type of organism. Recognition Site cut The restriction enzyme Eco. RI cuts here GAATTC DNA CTTAAG cut

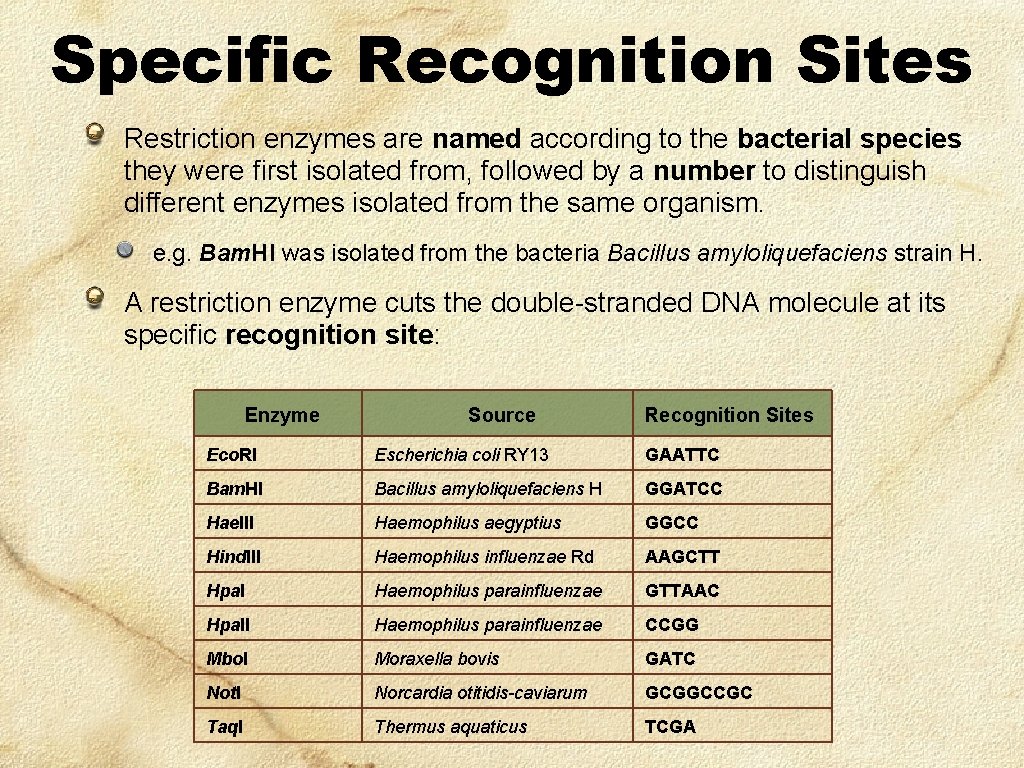
Specific Recognition Sites Restriction enzymes are named according to the bacterial species they were first isolated from, followed by a number to distinguish different enzymes isolated from the same organism. e. g. Bam. HI was isolated from the bacteria Bacillus amyloliquefaciens strain H. A restriction enzyme cuts the double-stranded DNA molecule at its specific recognition site: Enzyme Source Recognition Sites Eco. RI Escherichia coli RY 13 GAATTC Bam. HI Bacillus amyloliquefaciens H GGATCC Hae. III Haemophilus aegyptius GGCC Hind. III Haemophilus influenzae Rd AAGCTT Hpal Haemophilus parainfluenzae GTTAAC Hpa. II Haemophilus parainfluenzae CCGG Mbo. I Moraxella bovis GATC Not. I Norcardia otitidis-caviarum GCGGCCGC Taq. I Thermus aquaticus TCGA

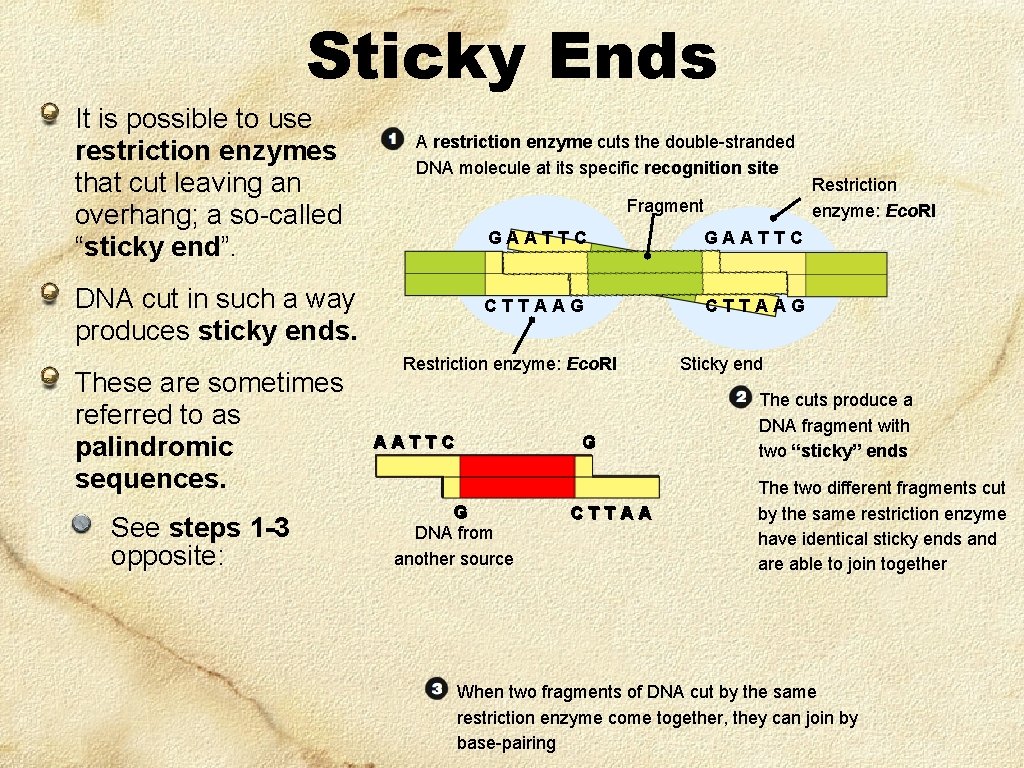
Sticky Ends It is possible to use restriction enzymes that cut leaving an overhang; a so-called “sticky end”. A restriction enzyme cuts the double-stranded DNA molecule at its specific recognition site Fragment DNA cut in such a way produces sticky ends. These are sometimes referred to as palindromic sequences. See steps 1 -3 opposite: GAATTC CTTAAG Restriction enzyme: Eco. RI AATTC G G DNA from another source CTTAA Restriction enzyme: Eco. RI Sticky end The cuts produce a DNA fragment with two “sticky” ends The two different fragments cut by the same restriction enzyme have identical sticky ends and are able to join together When two fragments of DNA cut by the same restriction enzyme come together, they can join by base-pairing

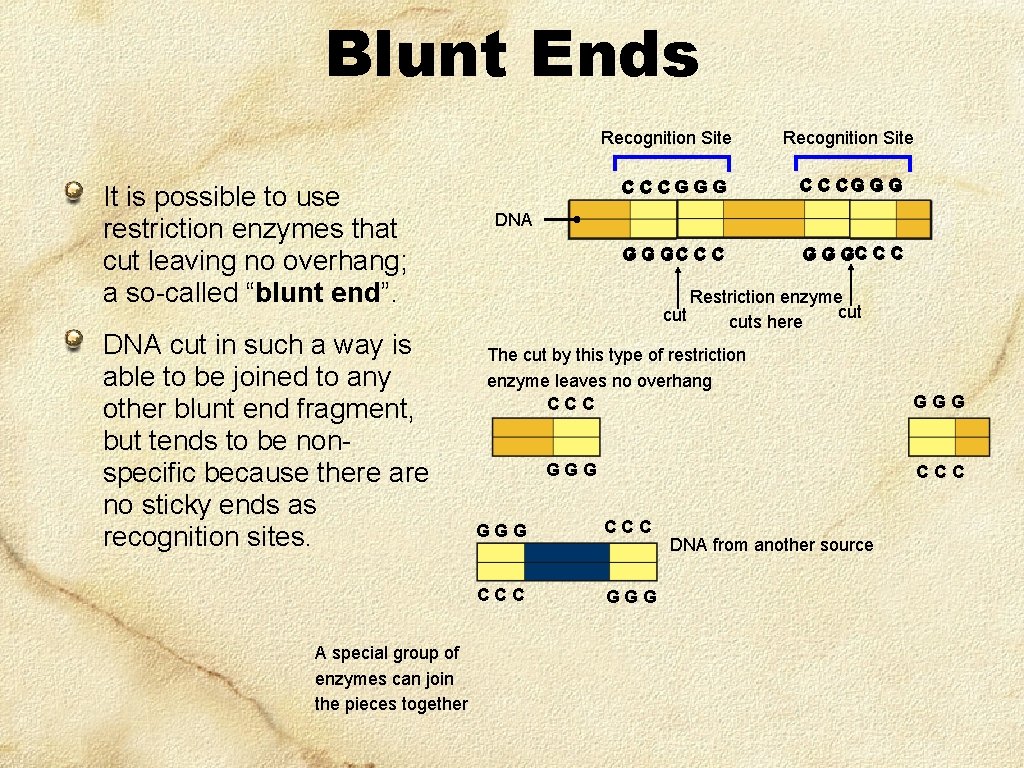
Blunt Ends Recognition Site It is possible to use restriction enzymes that cut leaving no overhang; a so-called “blunt end”. DNA cut in such a way is able to be joined to any other blunt end fragment, but tends to be nonspecific because there are no sticky ends as recognition sites. A special group of enzymes can join the pieces together Recognition Site C C CG G GC C C DNA Restriction enzyme cut cuts here The cut by this type of restriction enzyme leaves no overhang CCC GGG CCC GGG DNA from another source

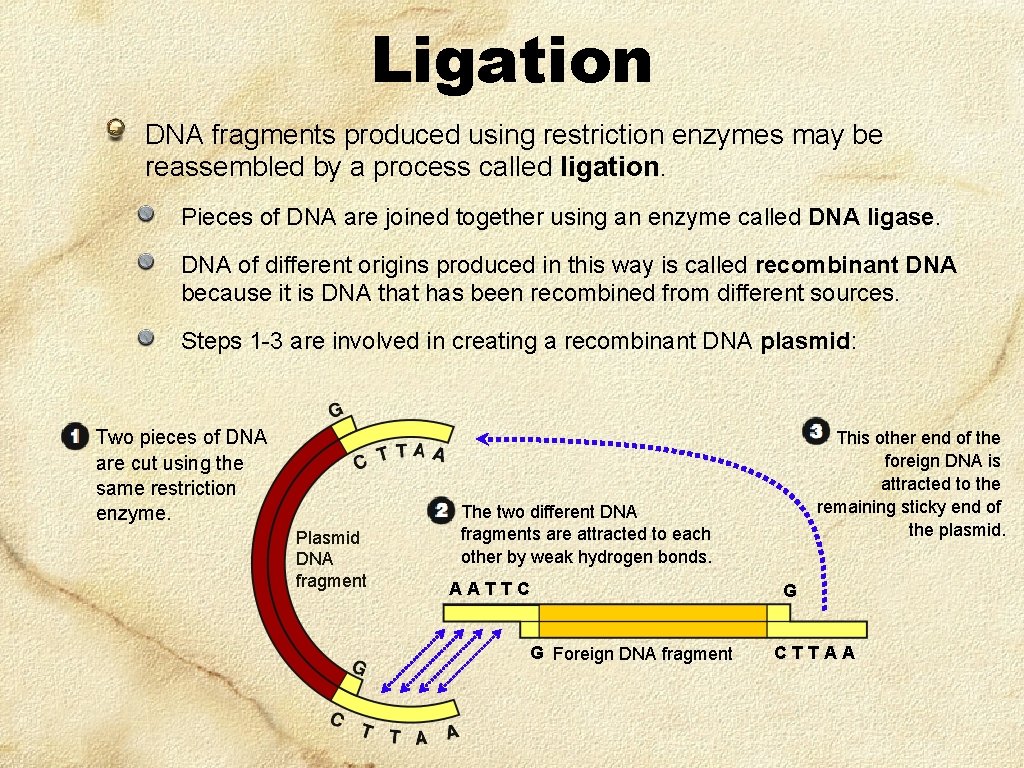
Ligation DNA fragments produced using restriction enzymes may be reassembled by a process called ligation. Pieces of DNA are joined together using an enzyme called DNA ligase. DNA of different origins produced in this way is called recombinant DNA because it is DNA that has been recombined from different sources. Steps 1 -3 are involved in creating a recombinant DNA plasmid: Two pieces of DNA are cut using the same restriction enzyme. Plasmid DNA fragment This other end of the foreign DNA is attracted to the remaining sticky end of the plasmid. The two different DNA fragments are attracted to each other by weak hydrogen bonds. AATTC G Foreign DNA fragment G CTTAA

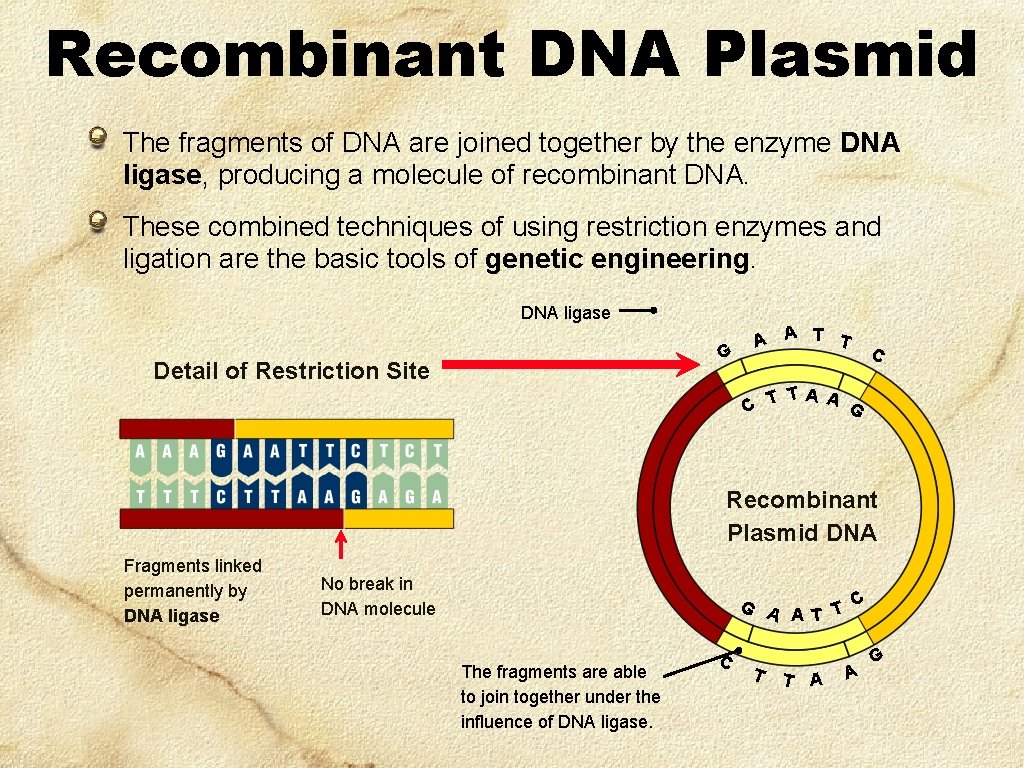
Recombinant DNA Plasmid The fragments of DNA are joined together by the enzyme DNA ligase, producing a molecule of recombinant DNA. These combined techniques of using restriction enzymes and ligation are the basic tools of genetic engineering. DNA ligase G Detail of Restriction Site A A T T C TA A G C T Recombinant Plasmid DNA Fragments linked permanently by DNA ligase No break in DNA molecule C G A T The fragments are able to join together under the influence of DNA ligase. C T T A A G

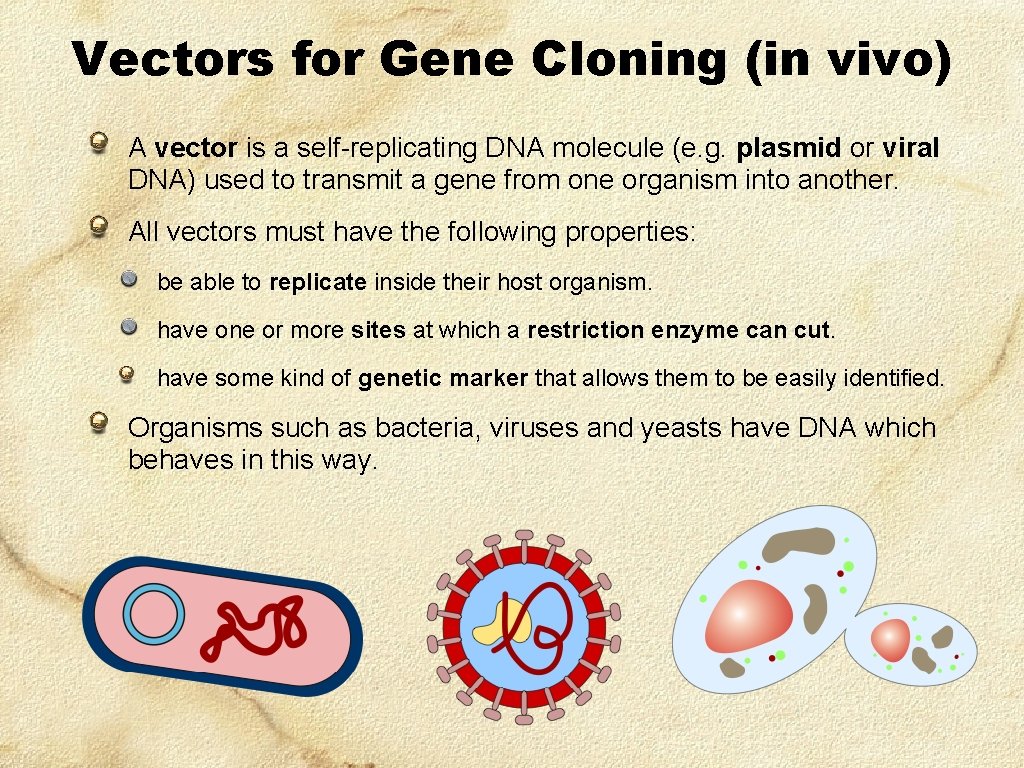
Vectors for Gene Cloning (in vivo) A vector is a self-replicating DNA molecule (e. g. plasmid or viral DNA) used to transmit a gene from one organism into another. All vectors must have the following properties: be able to replicate inside their host organism. have one or more sites at which a restriction enzyme can cut. have some kind of genetic marker that allows them to be easily identified. Organisms such as bacteria, viruses and yeasts have DNA which behaves in this way.

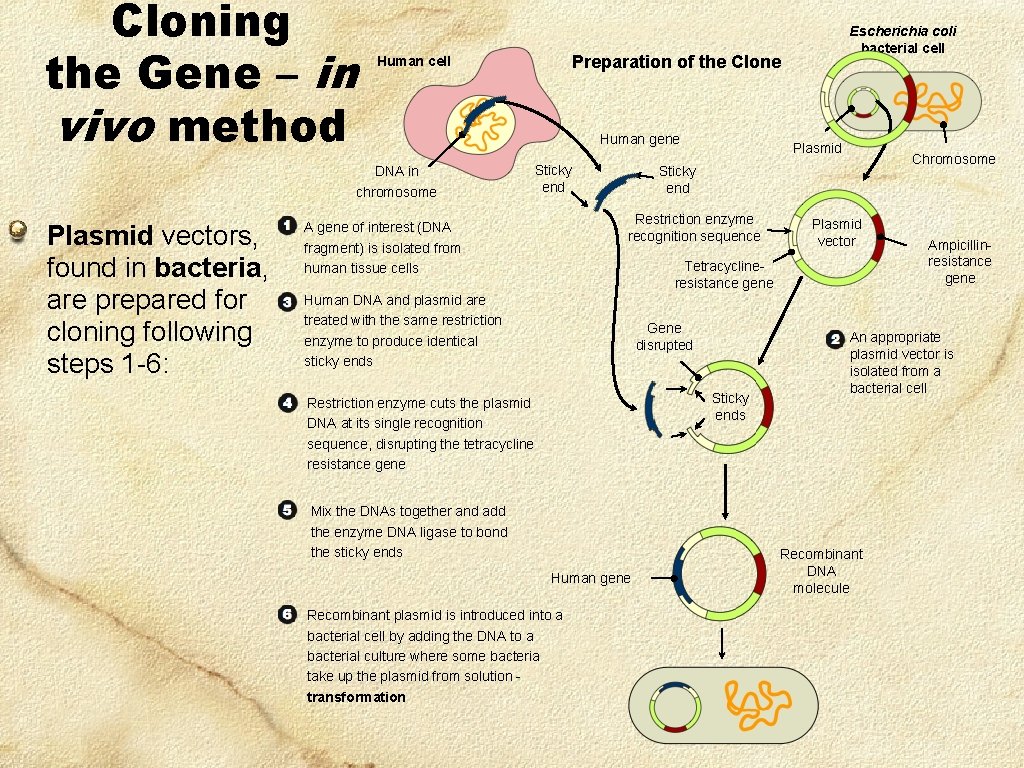
Cloning the Gene – in vivo method Plasmid vectors, found in bacteria, are prepared for cloning following steps 1 -6: Preparation of the Clone Human cell DNA in chromosome Escherichia coli bacterial cell Human gene Sticky end Plasmid Restriction enzyme recognition sequence A gene of interest (DNA fragment) is isolated from human tissue cells Chromosome Sticky end Plasmid vector Tetracyclineresistance gene Human DNA and plasmid are treated with the same restriction enzyme to produce identical sticky ends Gene disrupted Sticky ends Restriction enzyme cuts the plasmid DNA at its single recognition sequence, disrupting the tetracycline resistance gene Mix the DNAs together and add the enzyme DNA ligase to bond the sticky ends Human gene Recombinant plasmid is introduced into a bacterial cell by adding the DNA to a bacterial culture where some bacteria take up the plasmid from solution transformation Ampicillinresistance gene An appropriate plasmid vector is isolated from a bacterial cell Recombinant DNA molecule

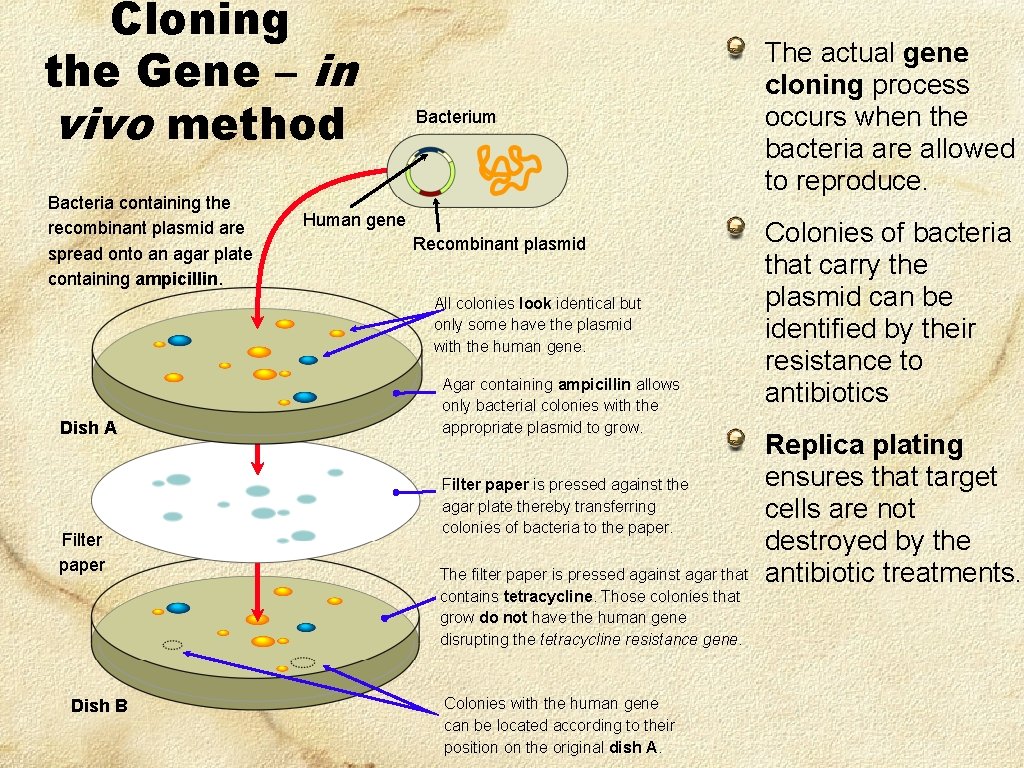
Cloning the Gene – in vivo method Bacteria containing the recombinant plasmid are spread onto an agar plate containing ampicillin. Bacterium Human gene Recombinant plasmid All colonies look identical but only some have the plasmid with the human gene. Dish A Filter paper Dish B Agar containing ampicillin allows only bacterial colonies with the appropriate plasmid to grow. Filter paper is pressed against the agar plate thereby transferring colonies of bacteria to the paper. The filter paper is pressed against agar that contains tetracycline. Those colonies that grow do not have the human gene disrupting the tetracycline resistance gene. Colonies with the human gene can be located according to their position on the original dish A. The actual gene cloning process occurs when the bacteria are allowed to reproduce. Colonies of bacteria that carry the plasmid can be identified by their resistance to antibiotics Replica plating ensures that target cells are not destroyed by the antibiotic treatments.

Gene Markers Using antibiotic-resistance genes as markers is, however, a rather out-dated technology. Geneticists now mainly use 2 other methods to identify the recombinant bacterial cells 1. Fluorescent markers In January 2001, for the first time scientists modified the DNA of a primate. They inserted a fluorescent gene from a jellyfish into the DNA of an unfertilized primate egg. This resulted in the birth of “ANDi” (“inserted DNA” backwards), the first transgenic primate (a Rhesus monkey). Now, the gene to be cloned can be inserted into a GFP (green fluorescent protein) gene – bacterial colonies that do not “glow” are the ones that have the desired gene. 2. Enzyme markers The gene is inserted into a gene for the production of lactase and the colony is then grown in a medium that changes colour if lactase is produced. Bacterial colonies where the growth medium remains colourless are the ones with the desired gene

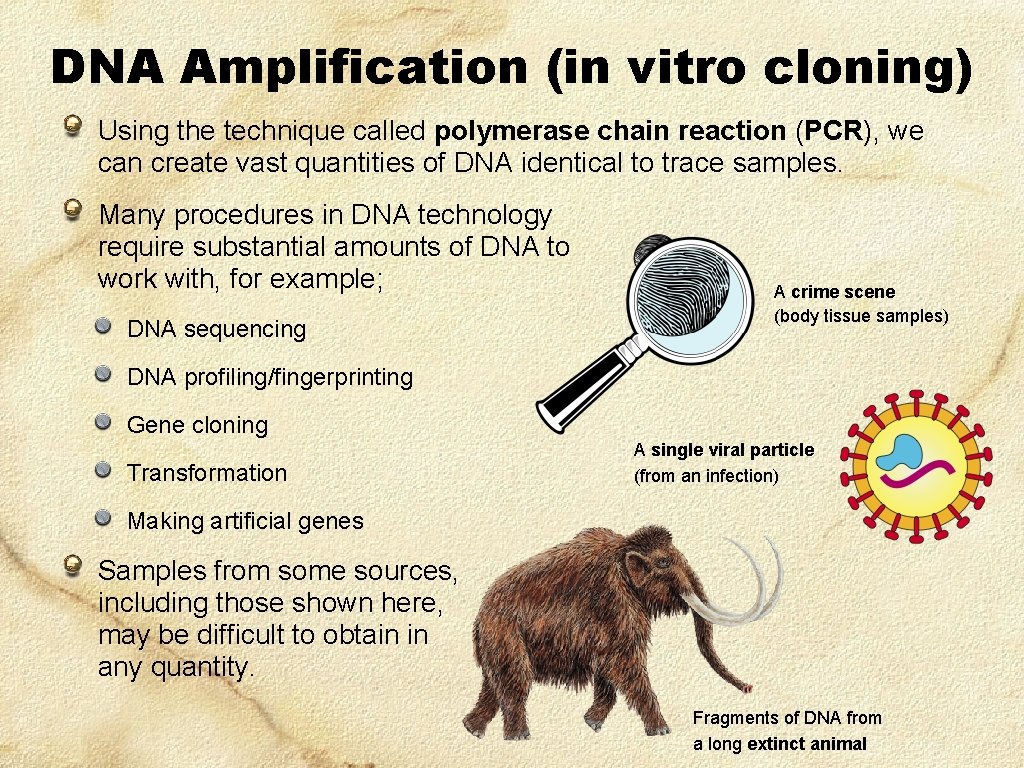
DNA Amplification (in vitro cloning) Using the technique called polymerase chain reaction (PCR), we can create vast quantities of DNA identical to trace samples. Many procedures in DNA technology require substantial amounts of DNA to work with, for example; DNA sequencing A crime scene (body tissue samples) DNA profiling/fingerprinting Gene cloning Transformation A single viral particle (from an infection) Making artificial genes Samples from some sources, including those shown here, may be difficult to obtain in any quantity. Fragments of DNA from a long extinct animal

PCR Equipment Amplification of DNA can be carried out with simple-to-use PCR machines called thermal cyclers (shown below). Thermal cyclers are in common use in the biology departments of universities as well as other kinds of research and analytical laboratories.

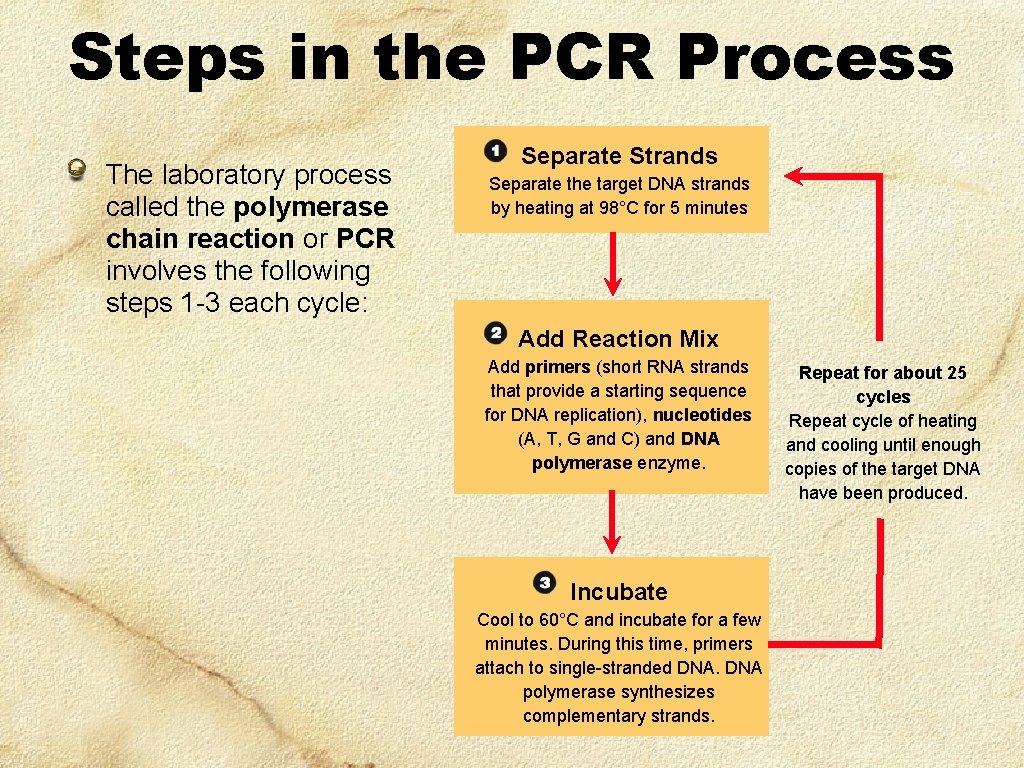
Steps in the PCR Process The laboratory process called the polymerase chain reaction or PCR involves the following steps 1 -3 each cycle: Separate Strands Separate the target DNA strands by heating at 98°C for 5 minutes Add Reaction Mix Add primers (short RNA strands that provide a starting sequence for DNA replication), nucleotides (A, T, G and C) and DNA polymerase enzyme. Incubate Cool to 60°C and incubate for a few minutes. During this time, primers attach to single-stranded DNA polymerase synthesizes complementary strands. Repeat for about 25 cycles Repeat cycle of heating and cooling until enough copies of the target DNA have been produced.

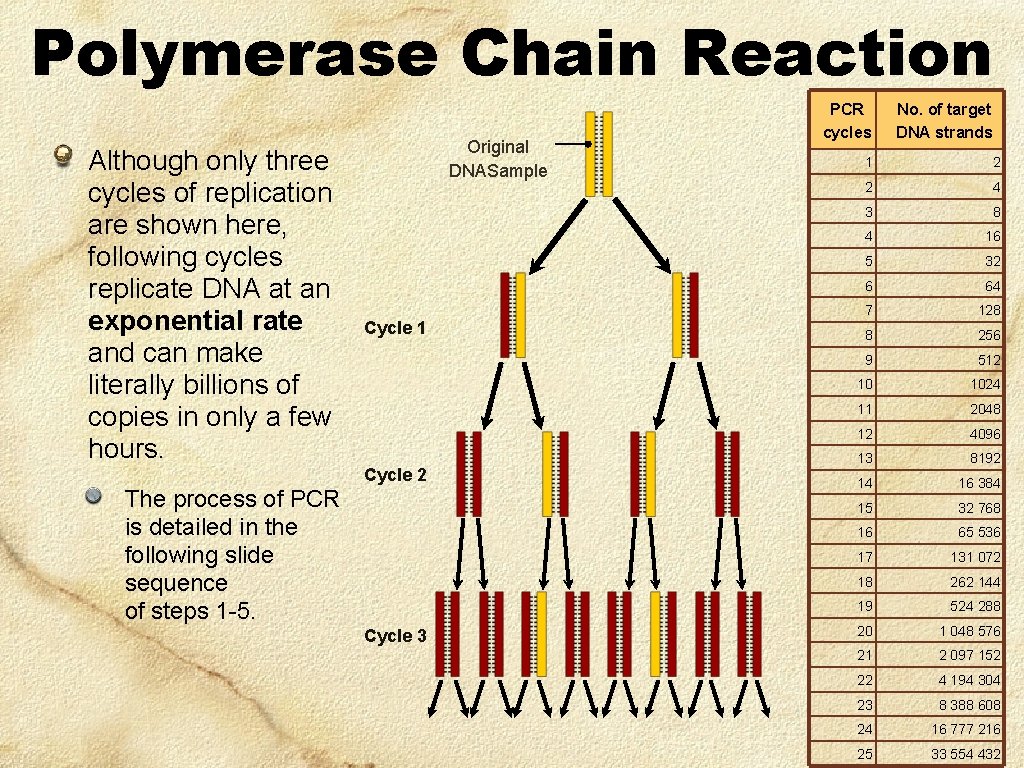
Polymerase Chain Reaction Although only three cycles of replication are shown here, following cycles replicate DNA at an exponential rate and can make literally billions of copies in only a few hours. The process of PCR is detailed in the following slide sequence of steps 1 -5. Original DNASample Cycle 1 Cycle 2 Cycle 3 PCR cycles No. of target DNA strands 1 2 2 4 3 8 4 16 5 32 6 64 7 128 8 256 9 512 10 1024 11 2048 12 4096 13 8192 14 16 384 15 32 768 16 65 536 17 131 072 18 262 144 19 524 288 20 1 048 576 21 2 097 152 22 4 194 304 23 8 388 608 24 16 777 216 25 33 554 432

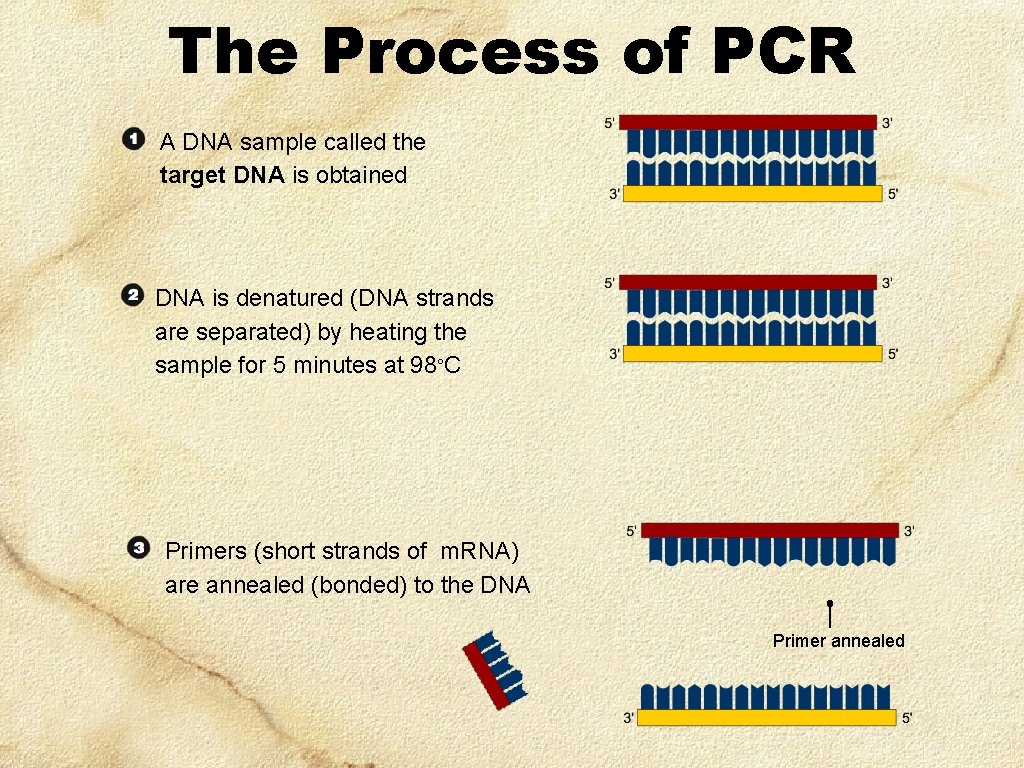
The Process of PCR A DNA sample called the target DNA is obtained DNA is denatured (DNA strands are separated) by heating the sample for 5 minutes at 98 C Primers (short strands of m. RNA) are annealed (bonded) to the DNA Primer annealed

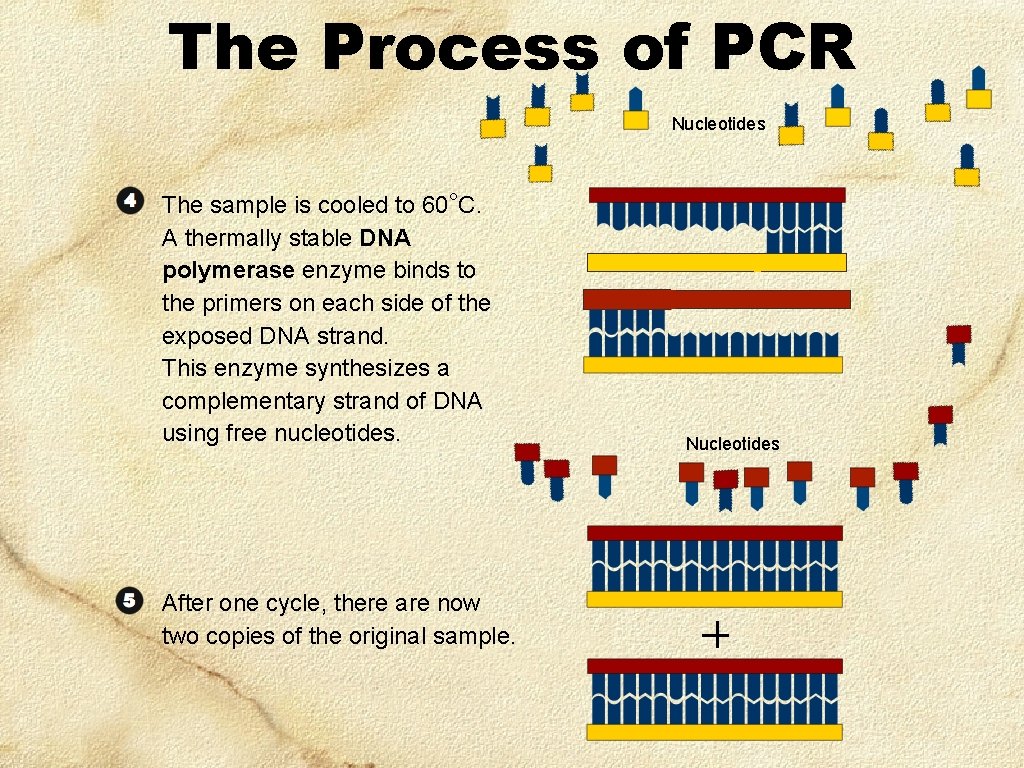
The Process of PCR Nucleotides The sample is cooled to 60°C. A thermally stable DNA polymerase enzyme binds to the primers on each side of the exposed DNA strand. This enzyme synthesizes a complementary strand of DNA using free nucleotides. After one cycle, there are now two copies of the original sample. Nucleotides

Gene Therapy Gene therapy refers to the use of gene technology to correct (supplementation) or replace defective genes. About two thirds of currently approved gene therapy procedures are targeting cancer, about one quarter aim to treat genetic disorders, such as cystic fibrosis, and the remainder are attempting to provide relief for infectious diseases. Gene therapy involving somatic cells may be therapeutic, but the genetic changes are not inherited, effects are short-lived and the process needs to be repeated regularly. The long term goal is the transfer of a gene into stem cells, rather than mature somatic cells. This will result in lifelong and permanent treatment. (although the cure is still not inherited) In the future, Germ-line therapy hopes to achieve the introduction of corrective genes into a fertilised egg cell. This will enable genetic corrections to be inherited. (currently prohibited)

Candidates for Gene Therapy The first attempt at gene therapy was when Ashanti De. Silva was treated for adenosine deaminase deficiency, which causes SCIDS, on 14 September 1990. She received new infusions of ADA restored cells every 1 -2 months for the first year, then every 3 -6 months thereafter. As a result, she has shown a clinical improvement and better immune function. Other genetic disorders that are candidates for clinical trials include: SCIDS (severe combined immune deficiency syndrome: "boy in the bubble") Cancers (including melanoma, breast, and colon cancer) Cystic fibrosis Hemophilia Rheumatoid arthritis Peripheral vascular disease Huntington’s Disease (an inherited sex linked enzyme disorder) Inherited high blood cholesterol

Medical Conditions Genetic material delivered to a patient’s cells could be used to treat a number of medical conditions such as: Restoring the function of a gene that has been lost as a result of mutation (i. e. possesses a harmful allele). By replacing missing genes or modifying faulty genes, it may be possible to treat genetic diseases. Rendering cells resistant to the toxic drugs used in the medical treatment of diseases. Also, killing abnormal cells such as those in cancerous tumors. Introduce genes that inhibit the reproduction of infectious agents such as viruses, bacteria, and endoparasites. There have been suggestions that the techniques of gene therapy may also be put to use to create “designer babies” that have traits selected by the parents.

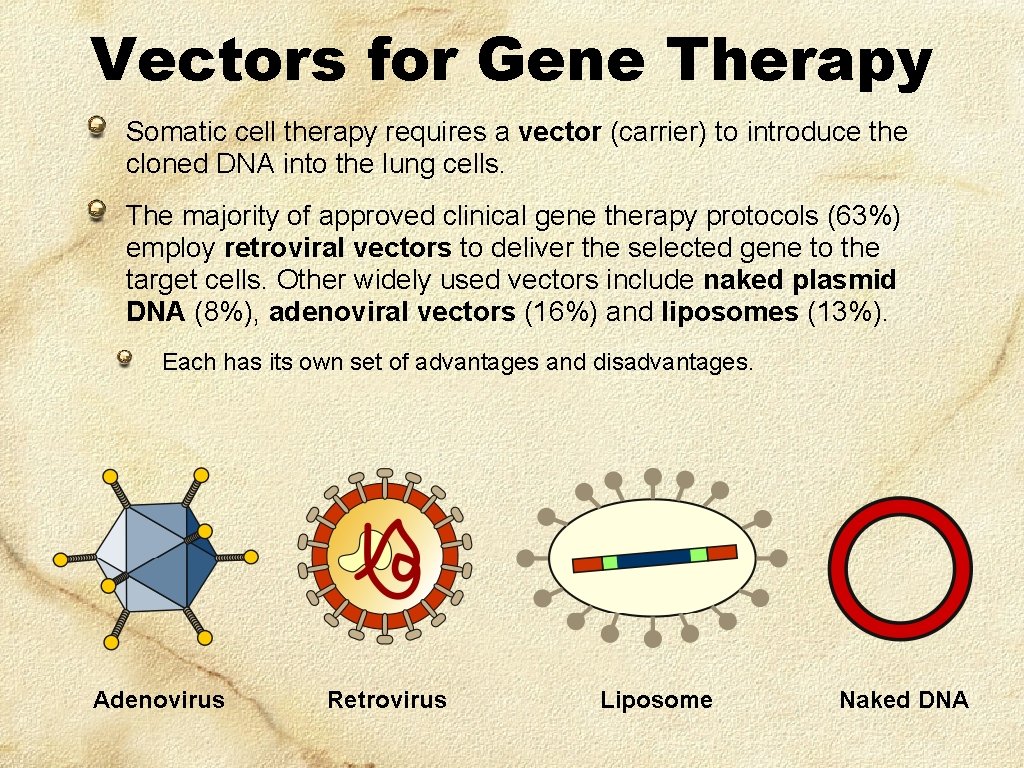
Vectors for Gene Therapy Somatic cell therapy requires a vector (carrier) to introduce the cloned DNA into the lung cells. The majority of approved clinical gene therapy protocols (63%) employ retroviral vectors to deliver the selected gene to the target cells. Other widely used vectors include naked plasmid DNA (8%), adenoviral vectors (16%) and liposomes (13%). Each has its own set of advantages and disadvantages. Adenovirus Retrovirus Liposome Naked DNA

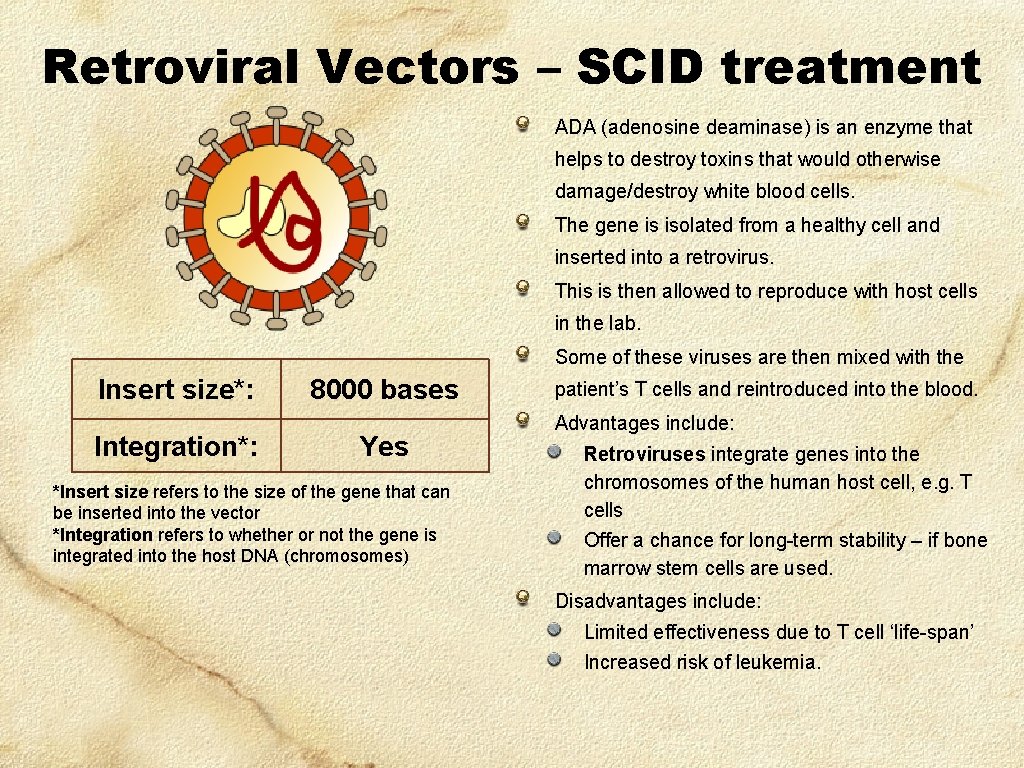
Retroviral Vectors – SCID treatment ADA (adenosine deaminase) is an enzyme that helps to destroy toxins that would otherwise damage/destroy white blood cells. The gene is isolated from a healthy cell and inserted into a retrovirus. This is then allowed to reproduce with host cells in the lab. Some of these viruses are then mixed with the Insert size*: Integration*: 8000 bases Yes *Insert size refers to the size of the gene that can be inserted into the vector *Integration refers to whether or not the gene is integrated into the host DNA (chromosomes) patient’s T cells and reintroduced into the blood. Advantages include: Retroviruses integrate genes into the chromosomes of the human host cell, e. g. T cells Offer a chance for long-term stability – if bone marrow stem cells are used. Disadvantages include: Limited effectiveness due to T cell ‘life-span’ Increased risk of leukemia.

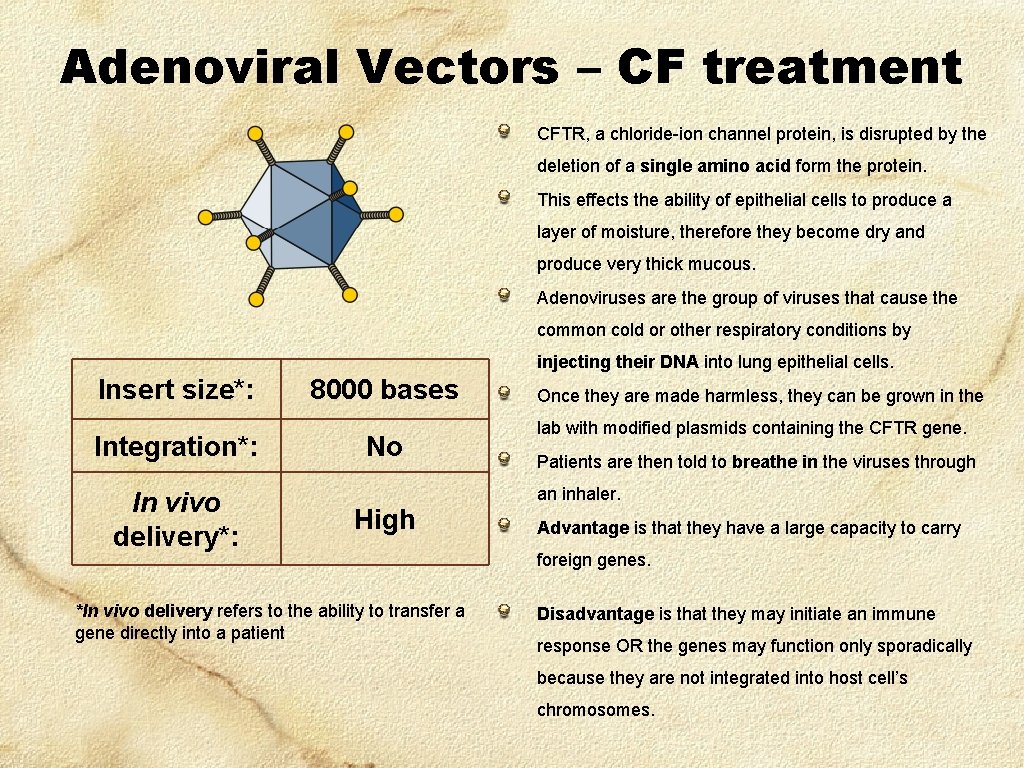
Adenoviral Vectors – CF treatment CFTR, a chloride-ion channel protein, is disrupted by the deletion of a single amino acid form the protein. This effects the ability of epithelial cells to produce a layer of moisture, therefore they become dry and produce very thick mucous. Adenoviruses are the group of viruses that cause the common cold or other respiratory conditions by injecting their DNA into lung epithelial cells. Insert size*: Integration*: In vivo delivery*: 8000 bases No High *In vivo delivery refers to the ability to transfer a gene directly into a patient Once they are made harmless, they can be grown in the lab with modified plasmids containing the CFTR gene. Patients are then told to breathe in the viruses through an inhaler. Advantage is that they have a large capacity to carry foreign genes. Disadvantage is that they may initiate an immune response OR the genes may function only sporadically because they are not integrated into host cell’s chromosomes.

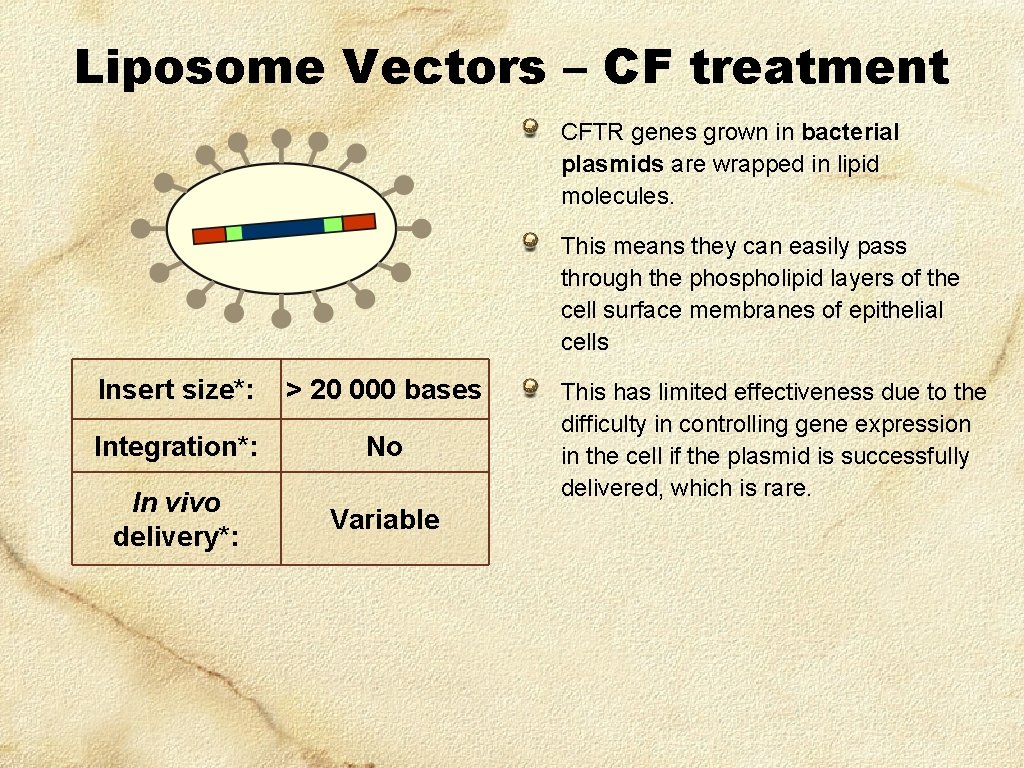
Liposome Vectors – CF treatment CFTR genes grown in bacterial plasmids are wrapped in lipid molecules. This means they can easily pass through the phospholipid layers of the cell surface membranes of epithelial cells Insert size*: > 20 000 bases Integration*: No In vivo delivery*: Variable This has limited effectiveness due to the difficulty in controlling gene expression in the cell if the plasmid is successfully delivered, which is rare.

Aerosols and Nebulizers Aerosols and nebulizers offer an effective, efficient delivery of the vector to the site of the target cells in the respiratory tract. Used in trials of gene therapy for cystic fibrosis (CF), but effective only on epithelial cells that can be reached by the aerosol. In these trials, liposomes containing normal CF genes were inhaled in a spray formulation but the overall results were disappointing.

Genome Analysis Genome analysis involves determining the exact order of all the millions of bases making up the DNA of an organism’s genome. It first requires the construction of a series of maps (both physical and genetic) of each chromosome at increasingly finer resolutions. Genome analysis must identify: All the genes present, their correct and exact location in the base sequence. The regions of DNA that control activity of the genes Chromosome Physical and Genetic Mapping Create a map showing location of gene markers on the chromosome Sequencing

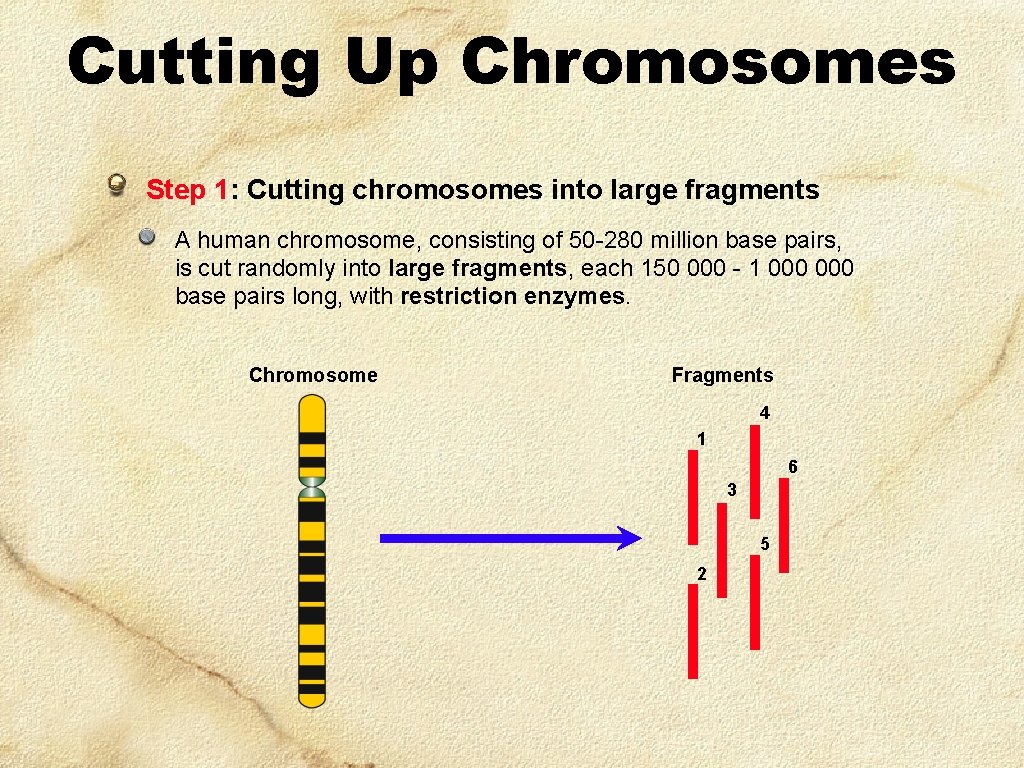
Cutting Up Chromosomes Step 1: Cutting chromosomes into large fragments A human chromosome, consisting of 50 -280 million base pairs, is cut randomly into large fragments, each 150 000 - 1 000 base pairs long, with restriction enzymes. Chromosome Fragments 4 1 6 3 5 2

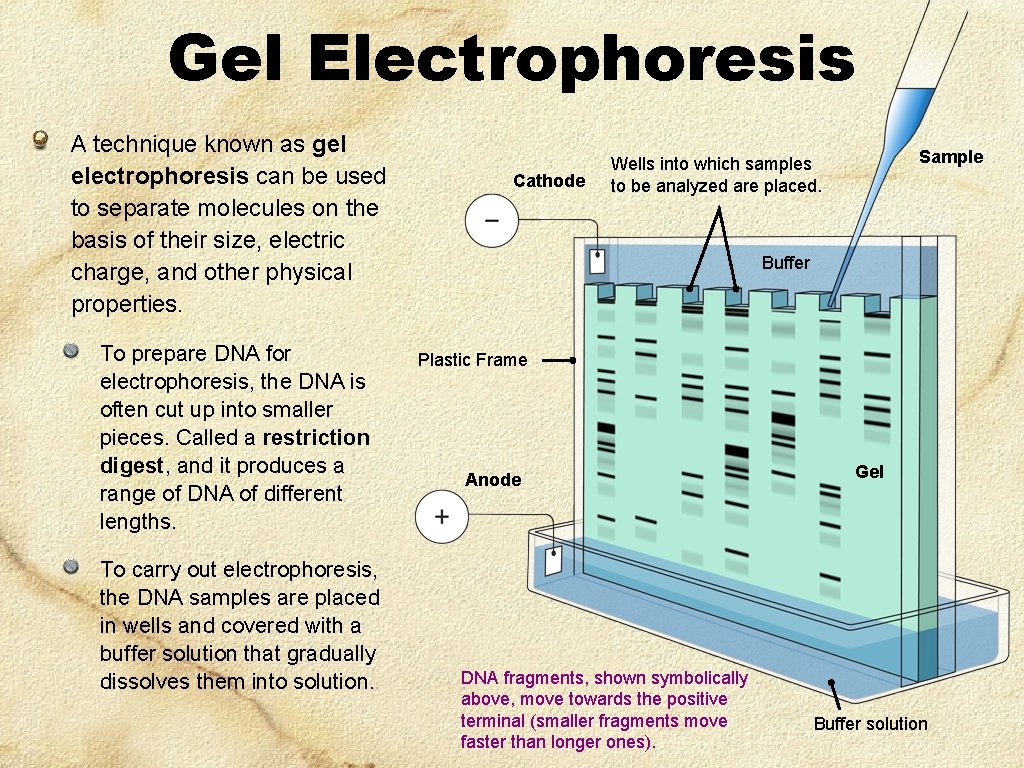
Gel Electrophoresis A technique known as gel electrophoresis can be used to separate molecules on the basis of their size, electric charge, and other physical properties. To prepare DNA for electrophoresis, the DNA is often cut up into smaller pieces. Called a restriction digest, and it produces a range of DNA of different lengths. To carry out electrophoresis, the DNA samples are placed in wells and covered with a buffer solution that gradually dissolves them into solution. Cathode Sample Wells into which samples to be analyzed are placed. Buffer Plastic Frame Anode DNA fragments, shown symbolically above, move towards the positive terminal (smaller fragments move faster than longer ones). Gel Buffer solution

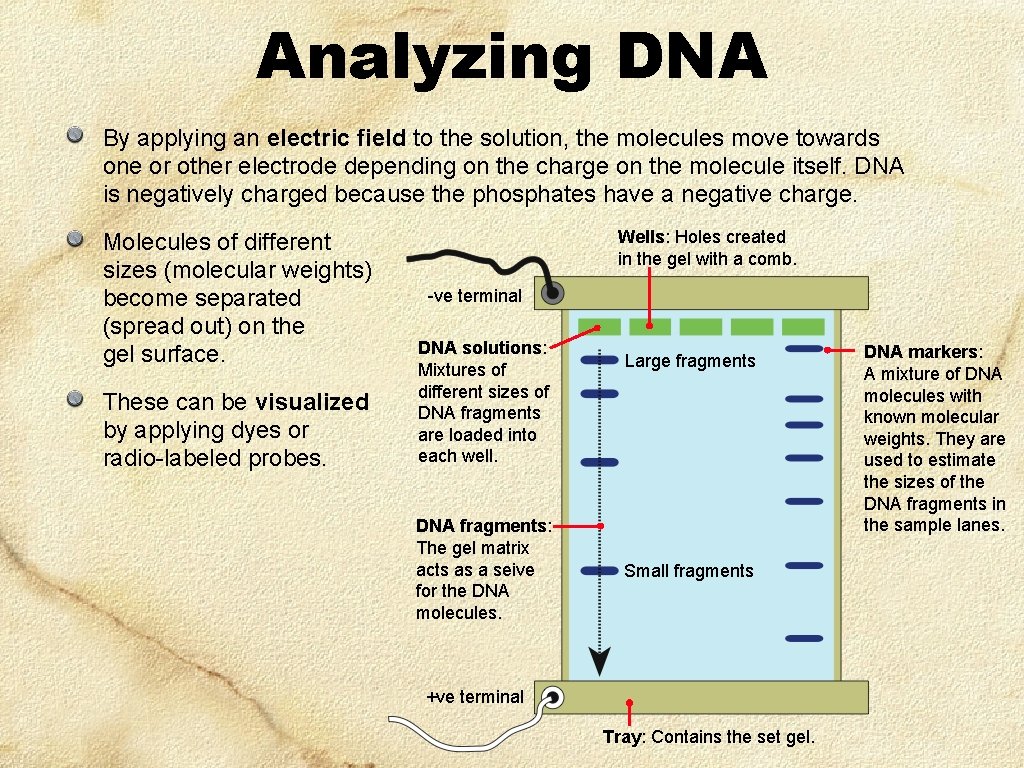
Analyzing DNA By applying an electric field to the solution, the molecules move towards one or other electrode depending on the charge on the molecule itself. DNA is negatively charged because the phosphates have a negative charge. Molecules of different sizes (molecular weights) become separated (spread out) on the gel surface. These can be visualized by applying dyes or radio-labeled probes. Wells: Holes created in the gel with a comb. -ve terminal DNA solutions: Mixtures of different sizes of DNA fragments are loaded into each well. DNA fragments: The gel matrix acts as a seive for the DNA molecules. Large fragments Small fragments +ve terminal Tray: Contains the set gel. DNA markers: A mixture of DNA molecules with known molecular weights. They are used to estimate the sizes of the DNA fragments in the sample lanes.

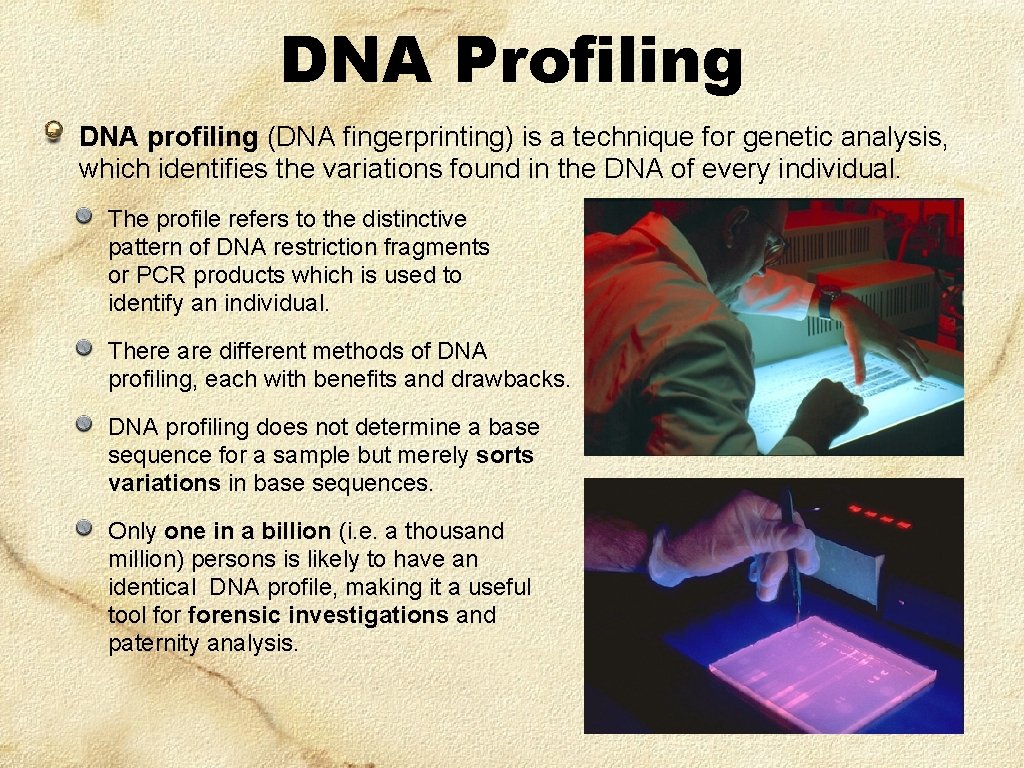
DNA Profiling DNA profiling (DNA fingerprinting) is a technique for genetic analysis, which identifies the variations found in the DNA of every individual. The profile refers to the distinctive pattern of DNA restriction fragments or PCR products which is used to identify an individual. There are different methods of DNA profiling, each with benefits and drawbacks. DNA profiling does not determine a base sequence for a sample but merely sorts variations in base sequences. Only one in a billion (i. e. a thousand million) persons is likely to have an identical DNA profile, making it a useful tool forensic investigations and paternity analysis.

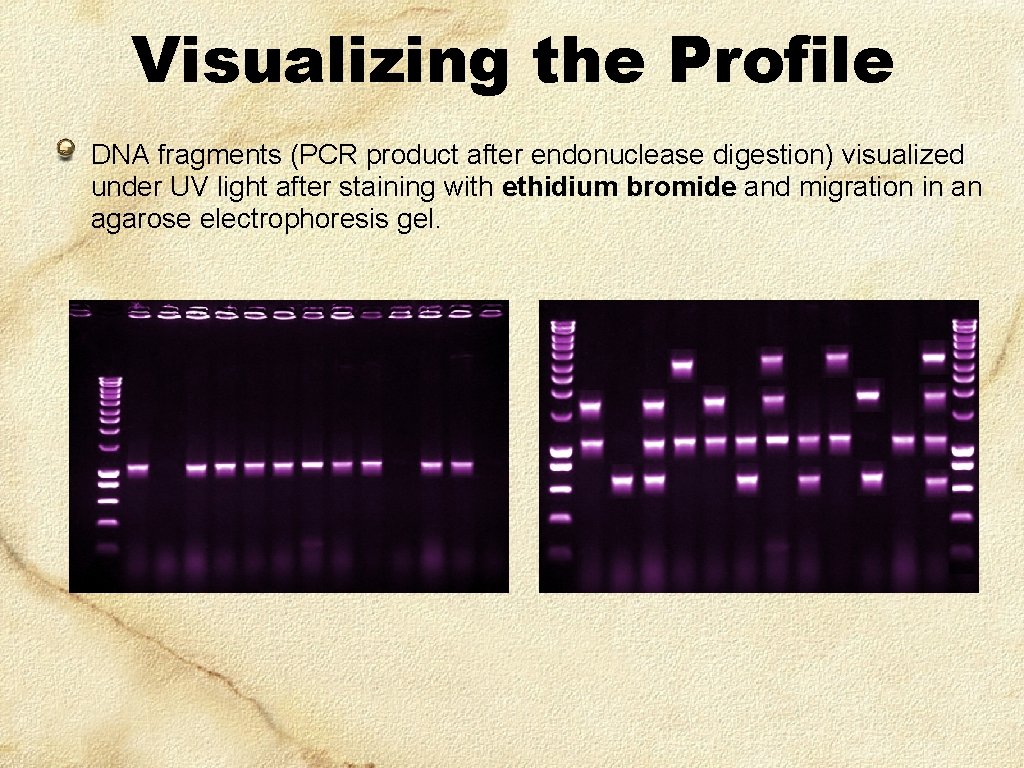
Visualizing the Profile DNA fragments (PCR product after endonuclease digestion) visualized under UV light after staining with ethidium bromide and migration in an agarose electrophoresis gel.

DNA Profiling Methods DNA profiling begins by extracting DNA from the cells in a sample of blood, saliva, semen, or other fluid or tissue. Two methods are commonly used. Both are based on the analysis of short repetitive sequences in the DNA. Profiling using probes (RFLP analysis) was the first profiling technique to be developed. Restriction enzymes are applied to a DNA sample and the DNA fragments are separated on a gel. Radioactive probes are used to label DNA fragments with complementary sequences. Profiling using PCR is newer technique which uses highly polymorphic regions of DNA that have short repeated sequences of DNA. These sequences are amplified using PCR and then separated on a gel. This technique is suitable when there is very little DNA available or the sample is old.

Uses of DNA Profiling DNA profiling can be used for investigating: the presence of a particular gene, such as cystic fibrosis) in a family. genetic relatedness of different organisms e. g. checking on pedigree in stock breeding programs. e. g. checking that captive populations of endangered species are not inbred.

DNA Profiling Using Probes The most commonly used manual method of DNA profiling is called the Southern blot. It uses the older technology of DNA probes and another type of repeat sequence to that used in profiling with PCR. The repeat sequences are called minisatellites or variable tandem repeats (VNTRs) and comprise repeating units of a few tens of nucleotides long. Equivalent sequences in different people have the same core sequence of 10 -15 bases (to which a DNA probe is attached), but thereafter the patterns vary considerable in length from one person to the next.

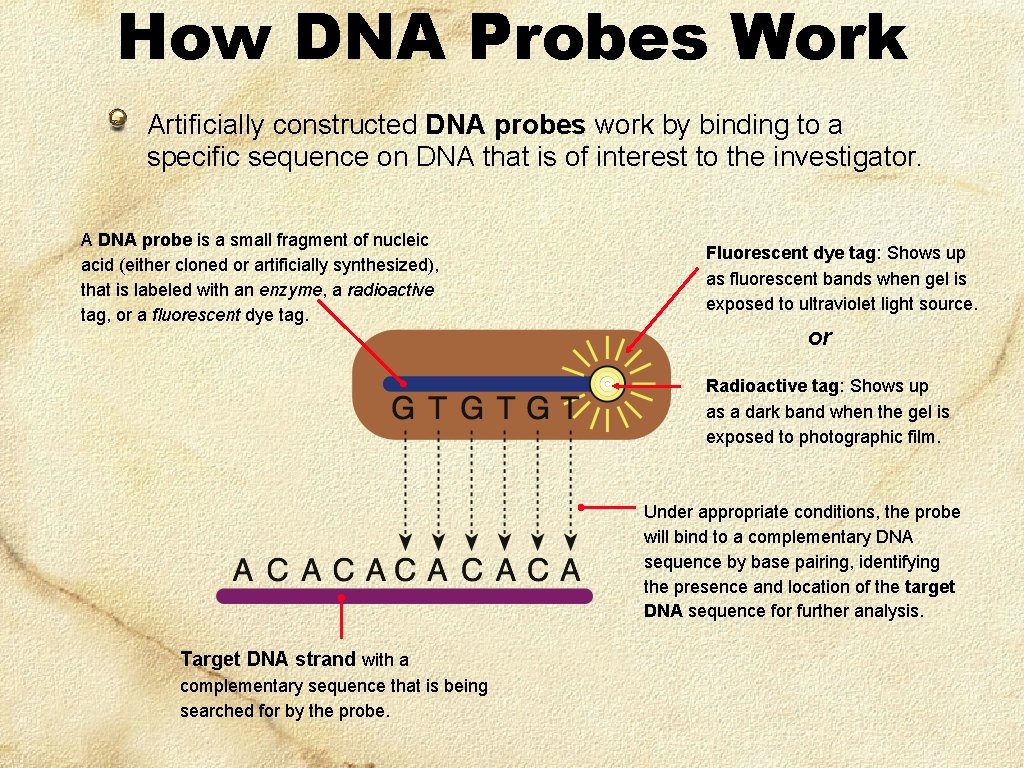
How DNA Probes Work Artificially constructed DNA probes work by binding to a specific sequence on DNA that is of interest to the investigator. A DNA probe is a small fragment of nucleic acid (either cloned or artificially synthesized), that is labeled with an enzyme, a radioactive tag, or a fluorescent dye tag. Fluorescent dye tag: Shows up as fluorescent bands when gel is exposed to ultraviolet light source. or Radioactive tag: Shows up as a dark band when the gel is exposed to photographic film. Under appropriate conditions, the probe will bind to a complementary DNA sequence by base pairing, identifying the presence and location of the target DNA sequence for further analysis. Target DNA strand with a complementary sequence that is being searched for by the probe.

What Gene Probes Do Gene probes may be used to search for: the presence of a specific allele of a gene (e. g. cystic fibrosis gene). the sequence of bases in a gene (HGP). the ‘genetic fingerprint’ of a person to tell them apart from others (e. g. paternity testing, forensic identification of suspects, the identification of bodies from plane crashes and exhumed graves).

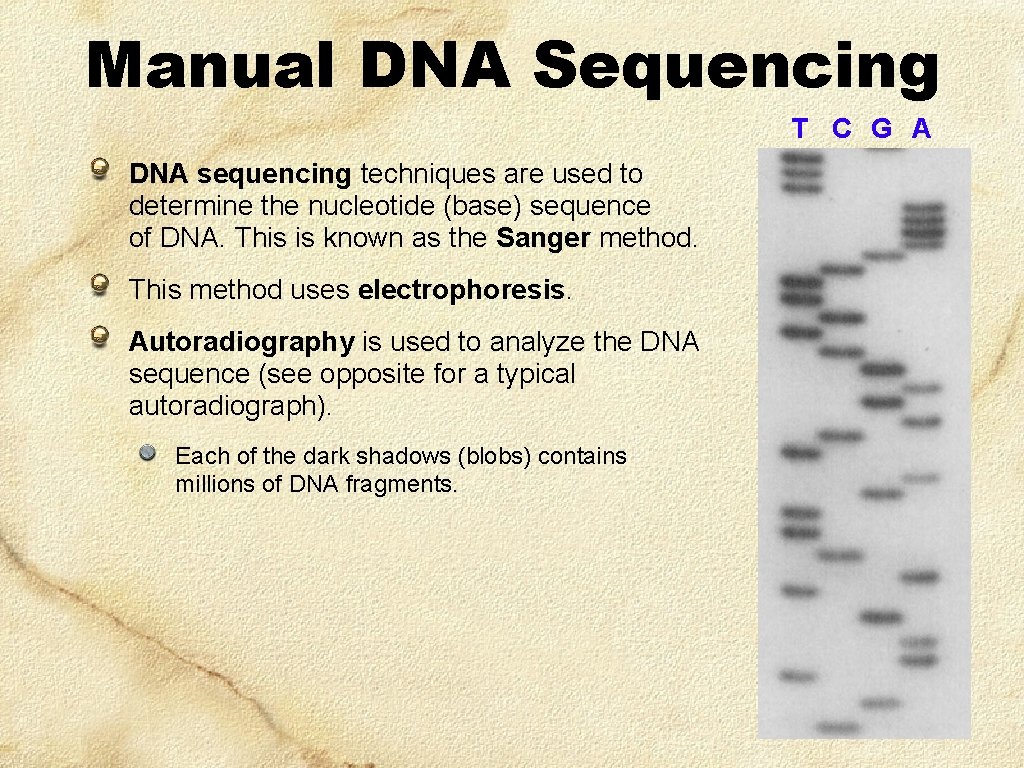
Manual DNA Sequencing T C G A DNA sequencing techniques are used to determine the nucleotide (base) sequence of DNA. This is known as the Sanger method. This method uses electrophoresis. Autoradiography is used to analyze the DNA sequence (see opposite for a typical autoradiograph). Each of the dark shadows (blobs) contains millions of DNA fragments.

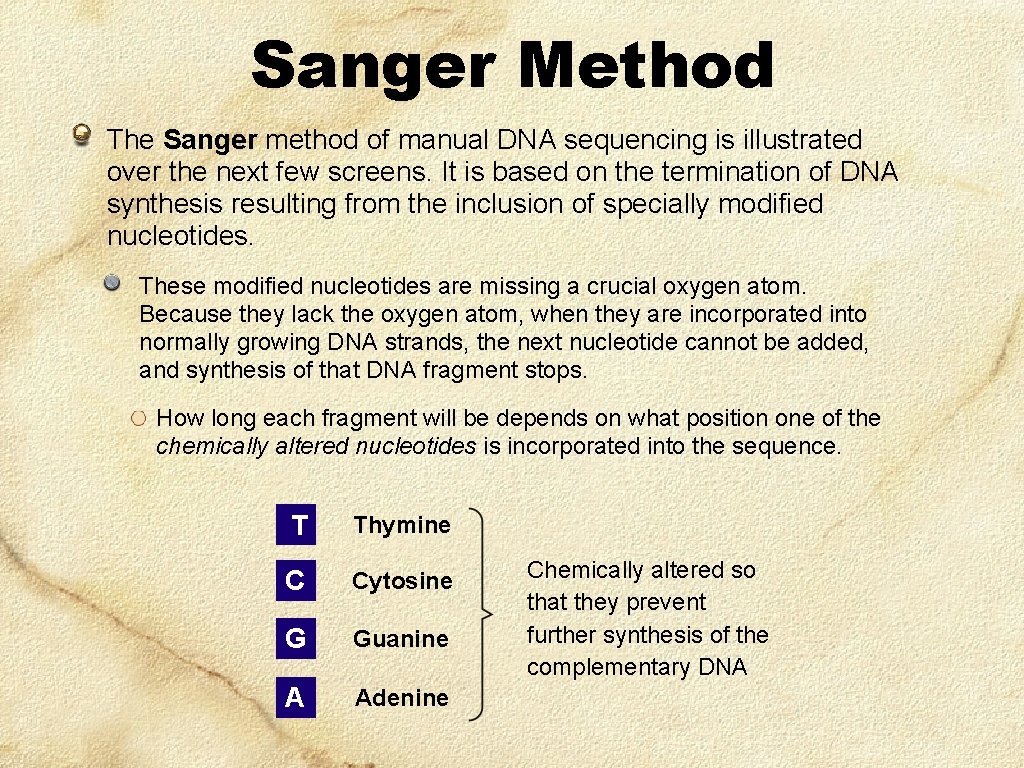
Sanger Method The Sanger method of manual DNA sequencing is illustrated over the next few screens. It is based on the termination of DNA synthesis resulting from the inclusion of specially modified nucleotides. These modified nucleotides are missing a crucial oxygen atom. Because they lack the oxygen atom, when they are incorporated into normally growing DNA strands, the next nucleotide cannot be added, and synthesis of that DNA fragment stops. How long each fragment will be depends on what position one of the chemically altered nucleotides is incorporated into the sequence. T Thymine C Cytosine G Guanine A Adenine Chemically altered so that they prevent further synthesis of the complementary DNA

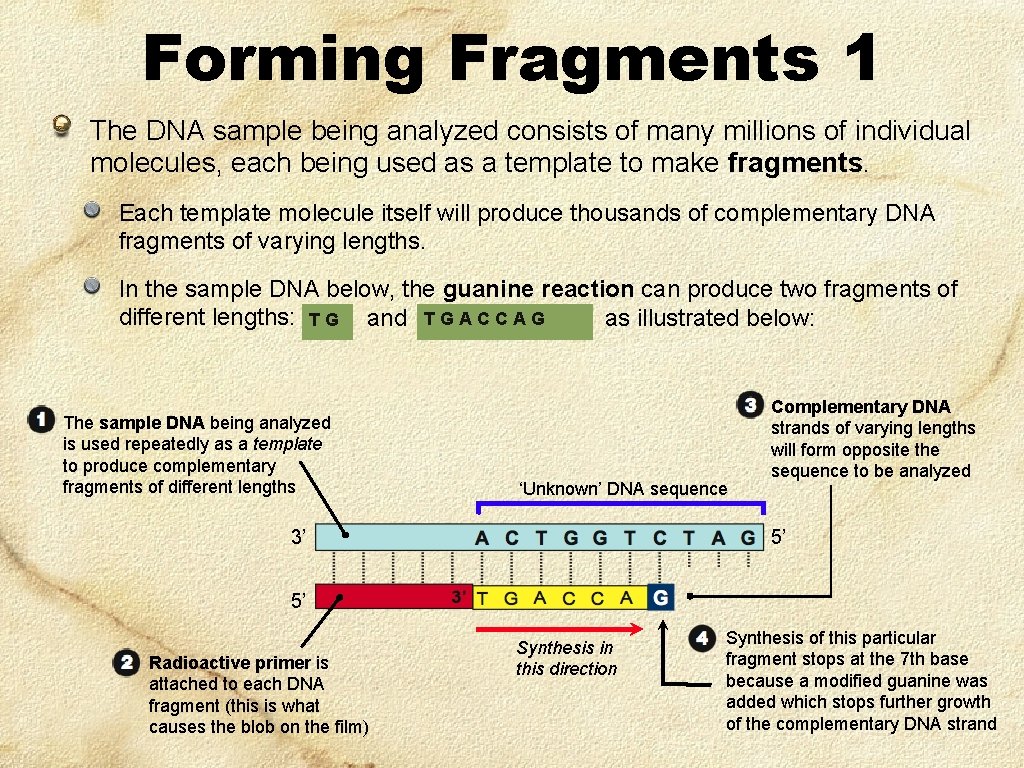
Forming Fragments 1 The DNA sample being analyzed consists of many millions of individual molecules, each being used as a template to make fragments. Each template molecule itself will produce thousands of complementary DNA fragments of varying lengths. In the sample DNA below, the guanine reaction can produce two fragments of different lengths: T G and T G A C C A G as illustrated below: The sample DNA being analyzed is used repeatedly as a template to produce complementary fragments of different lengths ‘Unknown’ DNA sequence 3’ Complementary DNA strands of varying lengths will form opposite the sequence to be analyzed 5’ 5’ Radioactive primer is attached to each DNA fragment (this is what causes the blob on the film) Synthesis in this direction Synthesis of this particular fragment stops at the 7 th base because a modified guanine was added which stops further growth of the complementary DNA strand

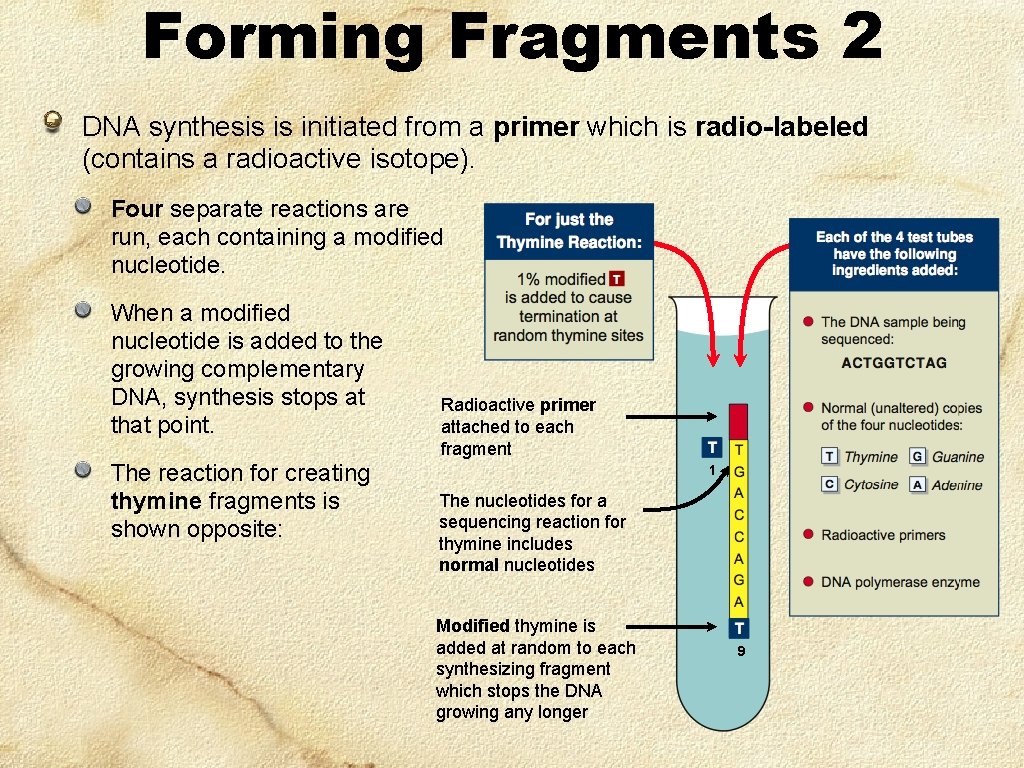
Forming Fragments 2 DNA synthesis is initiated from a primer which is radio-labeled (contains a radioactive isotope). Four separate reactions are run, each containing a modified nucleotide. When a modified nucleotide is added to the growing complementary DNA, synthesis stops at that point. The reaction for creating thymine fragments is shown opposite: Radioactive primer attached to each fragment 1 The nucleotides for a sequencing reaction for thymine includes normal nucleotides Modified thymine is added at random to each synthesizing fragment which stops the DNA growing any longer 9

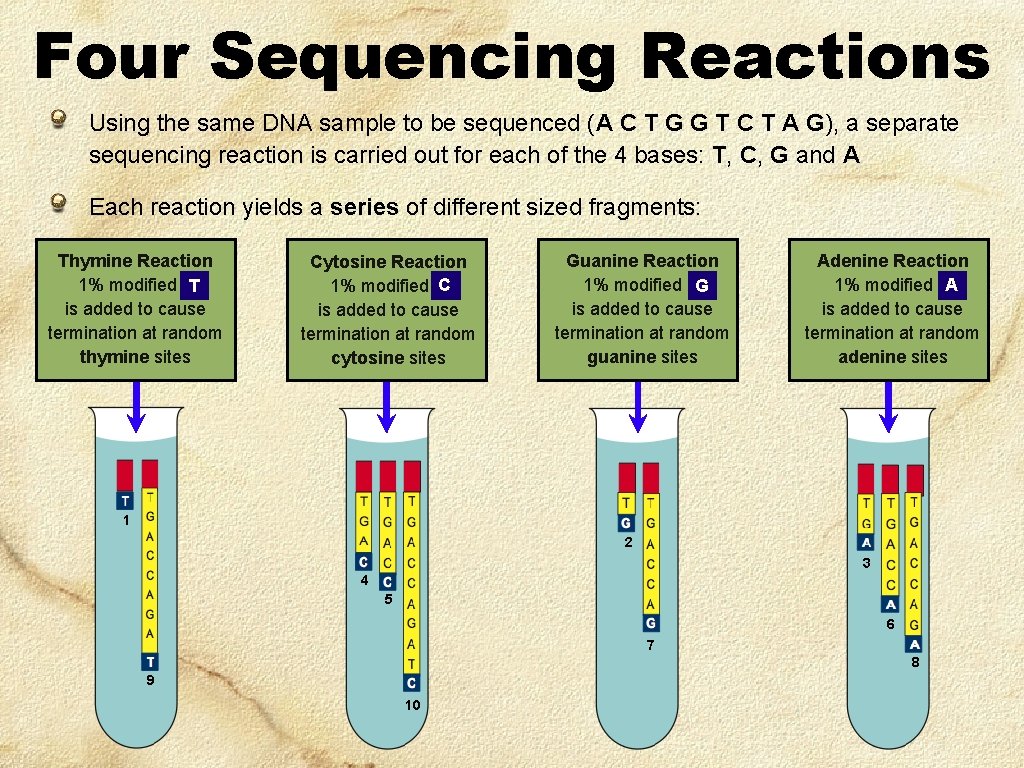
Four Sequencing Reactions Using the same DNA sample to be sequenced (A C T G G T C T A G), a separate sequencing reaction is carried out for each of the 4 bases: T, C, G and A Each reaction yields a series of different sized fragments: Thymine Reaction 1% modified TT is added to cause termination at random thymine sites Cytosine Reaction C 1% modified C is added to cause termination at random cytosine sites Guanine Reaction 1% modified GG is added to cause termination at random guanine sites Adenine Reaction 1% modified AA is added to cause termination at random adenine sites 1 2 3 4 5 6 7 8 9 10

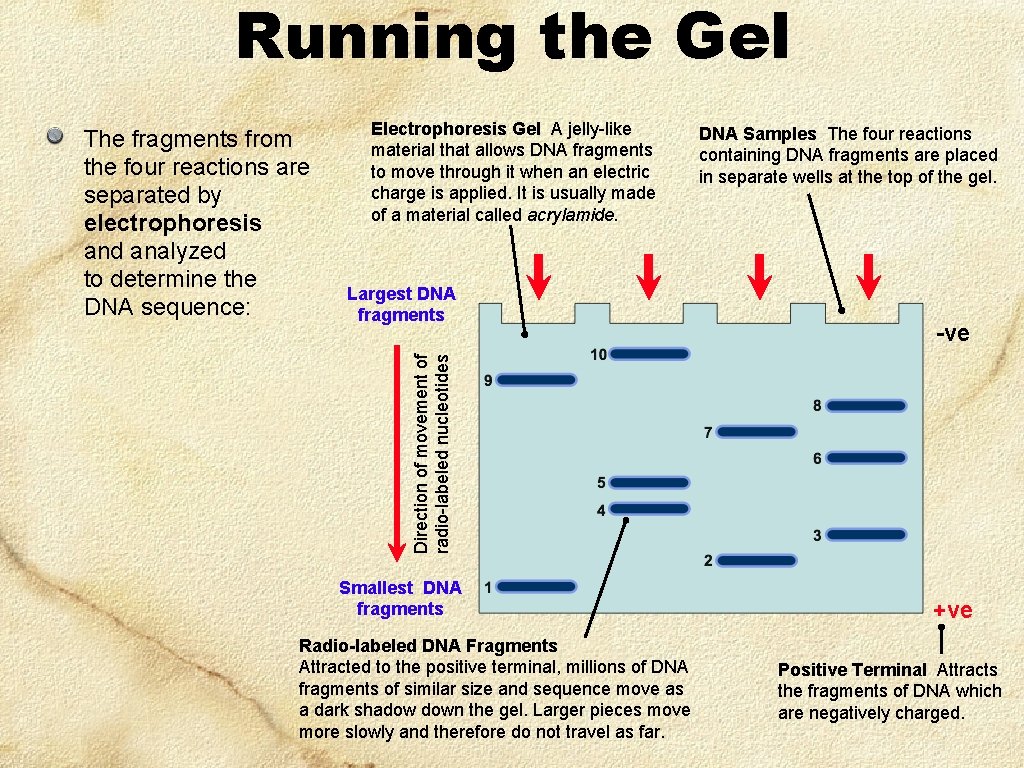
Running the Gel Electrophoresis Gel A jelly-like material that allows DNA fragments to move through it when an electric charge is applied. It is usually made of a material called acrylamide. Largest DNA fragments DNA Samples The four reactions containing DNA fragments are placed in separate wells at the top of the gel. -ve Direction of movement of radio-labeled nucleotides The fragments from the four reactions are separated by electrophoresis and analyzed to determine the DNA sequence: Smallest DNA fragments Radio-labeled DNA Fragments Attracted to the positive terminal, millions of DNA fragments of similar size and sequence move as a dark shadow down the gel. Larger pieces move more slowly and therefore do not travel as far. +ve Positive Terminal Attracts the fragments of DNA which are negatively charged.

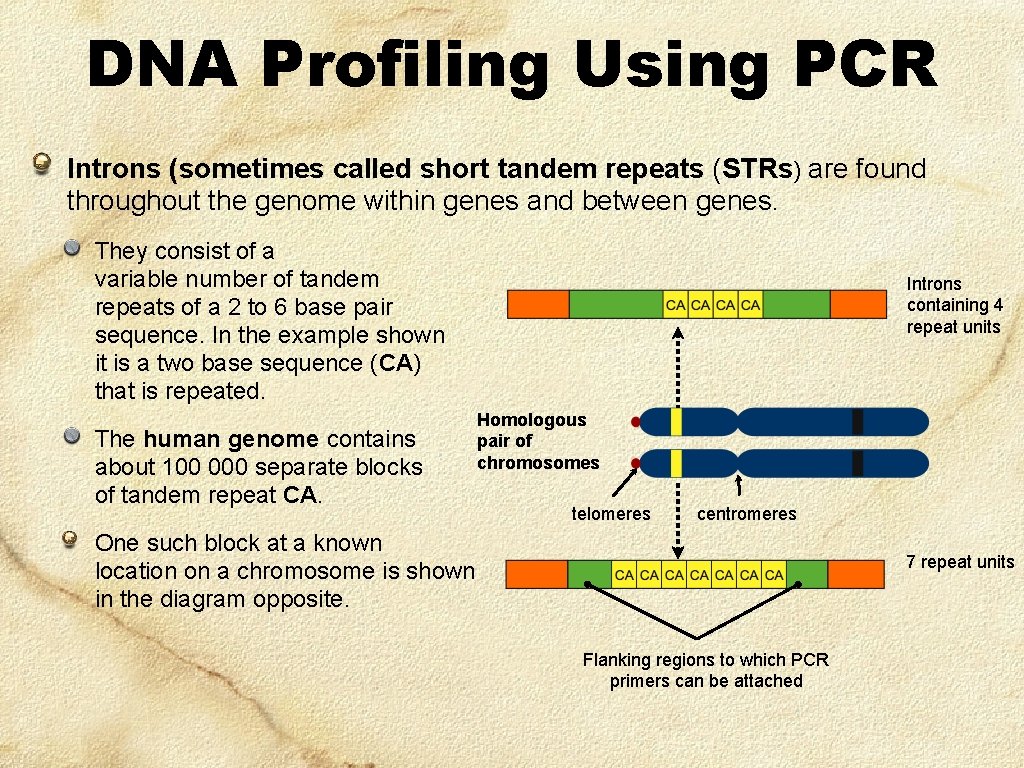
DNA Profiling Using PCR Introns (sometimes called short tandem repeats (STRs) are found throughout the genome within genes and between genes. They consist of a variable number of tandem repeats of a 2 to 6 base pair sequence. In the example shown it is a two base sequence (CA) that is repeated. The human genome contains about 100 000 separate blocks of tandem repeat CA. Introns containing 4 repeat units Homologous pair of chromosomes telomeres centromeres One such block at a known location on a chromosome is shown in the diagram opposite. 7 repeat units Flanking regions to which PCR primers can be attached

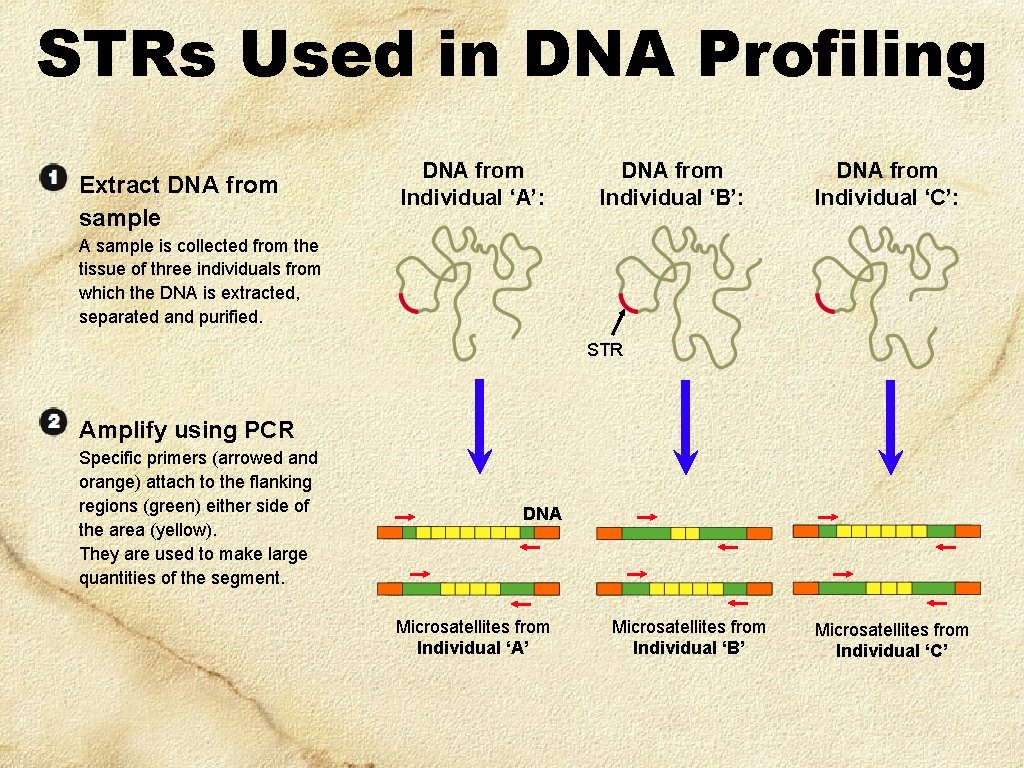
STRs Used in DNA Profiling Extract DNA from sample DNA from Individual ‘A’: DNA from Individual ‘B’: DNA from Individual ‘C’: A sample is collected from the tissue of three individuals from which the DNA is extracted, separated and purified. STR Amplify using PCR Specific primers (arrowed and orange) attach to the flanking regions (green) either side of the area (yellow). They are used to make large quantities of the segment. DNA Microsatellites from Individual ‘A’ Microsatellites from Individual ‘B’ Microsatellites from Individual ‘C’

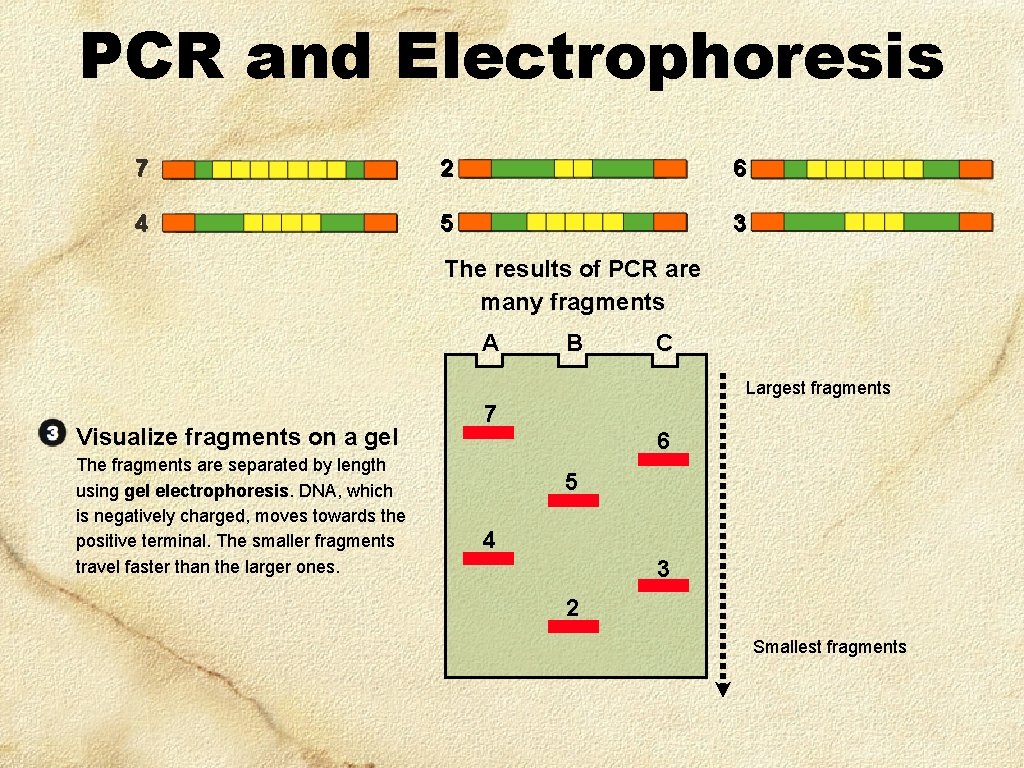
PCR and Electrophoresis 7 2 6 4 5 3 The results of PCR are many fragments A B C Largest fragments Visualize fragments on a gel The fragments are separated by length using gel electrophoresis. DNA, which is negatively charged, moves towards the positive terminal. The smaller fragments travel faster than the larger ones. 7 6 5 4 3 2 Smallest fragments

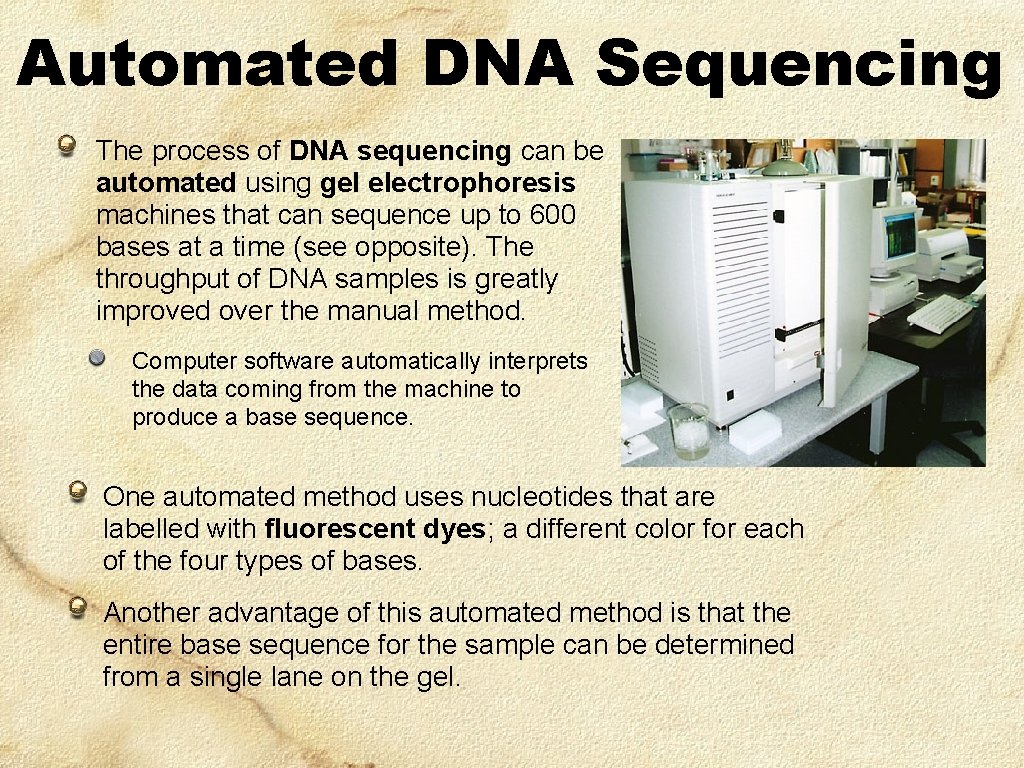
Automated DNA Sequencing The process of DNA sequencing can be automated using gel electrophoresis machines that can sequence up to 600 bases at a time (see opposite). The throughput of DNA samples is greatly improved over the manual method. Computer software automatically interprets the data coming from the machine to produce a base sequence. One automated method uses nucleotides that are labelled with fluorescent dyes; a different color for each of the four types of bases. Another advantage of this automated method is that the entire base sequence for the sample can be determined from a single lane on the gel.

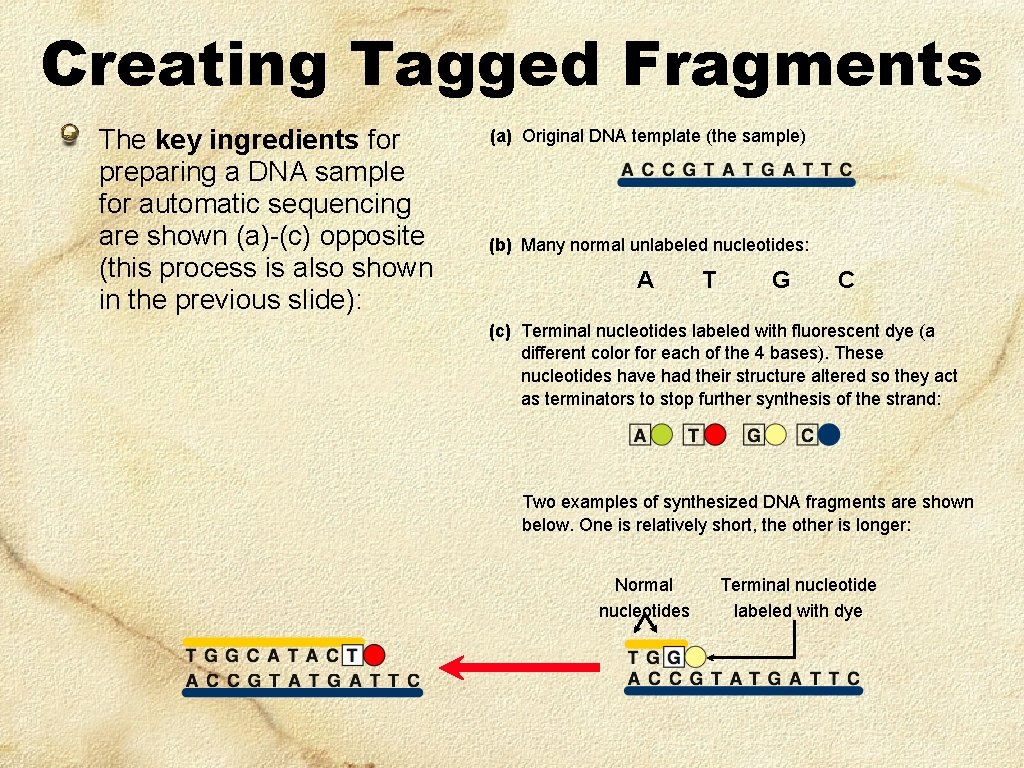
Creating Tagged Fragments The key ingredients for preparing a DNA sample for automatic sequencing are shown (a)-(c) opposite (this process is also shown in the previous slide): (a) Original DNA template (the sample) (b) Many normal unlabeled nucleotides: A T G C (c) Terminal nucleotides labeled with fluorescent dye (a different color for each of the 4 bases). These nucleotides have had their structure altered so they act as terminators to stop further synthesis of the strand: Two examples of synthesized DNA fragments are shown below. One is relatively short, the other is longer: Normal nucleotides Terminal nucleotide labeled with dye

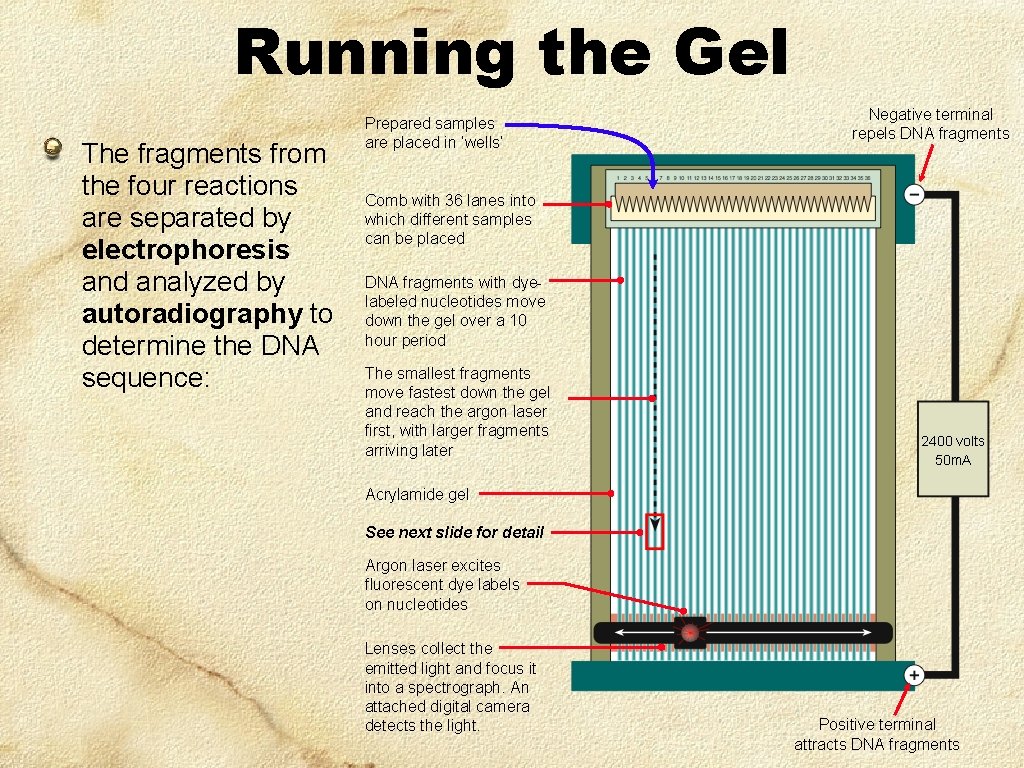
Running the Gel The fragments from the four reactions are separated by electrophoresis and analyzed by autoradiography to determine the DNA sequence: Prepared samples are placed in ‘wells’ Negative terminal repels DNA fragments Comb with 36 lanes into which different samples can be placed DNA fragments with dyelabeled nucleotides move down the gel over a 10 hour period The smallest fragments move fastest down the gel and reach the argon laser first, with larger fragments arriving later 2400 volts 50 m. A Acrylamide gel See next slide for detail Argon laser excites fluorescent dye labels on nucleotides Lenses collect the emitted light and focus it into a spectrograph. An attached digital camera detects the light. Positive terminal attracts DNA fragments

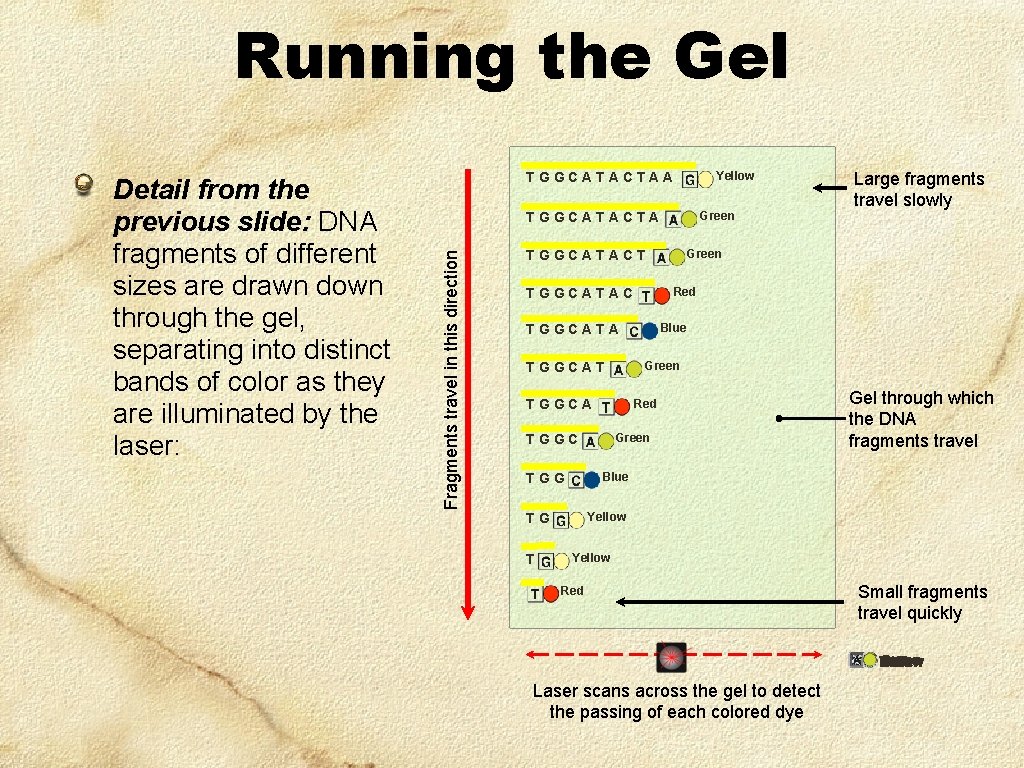
Running the Gel Green TGGCATACTA Fragments travel in this direction Detail from the previous slide: DNA fragments of different sizes are drawn down through the gel, separating into distinct bands of color as they are illuminated by the laser: Yellow TGGCATACTAA Green TGGCATACT Red TGGCATAC TGGCATA TGGCAT Red Green Gel through which the DNA fragments travel Blue TGG Yellow TG T Blue Green TGGCA TGGC Large fragments travel slowly Yellow Red Small fragments travel quickly Green Red Yellow Red Blue Laser scans across the gel to detect the passing of each colored dye

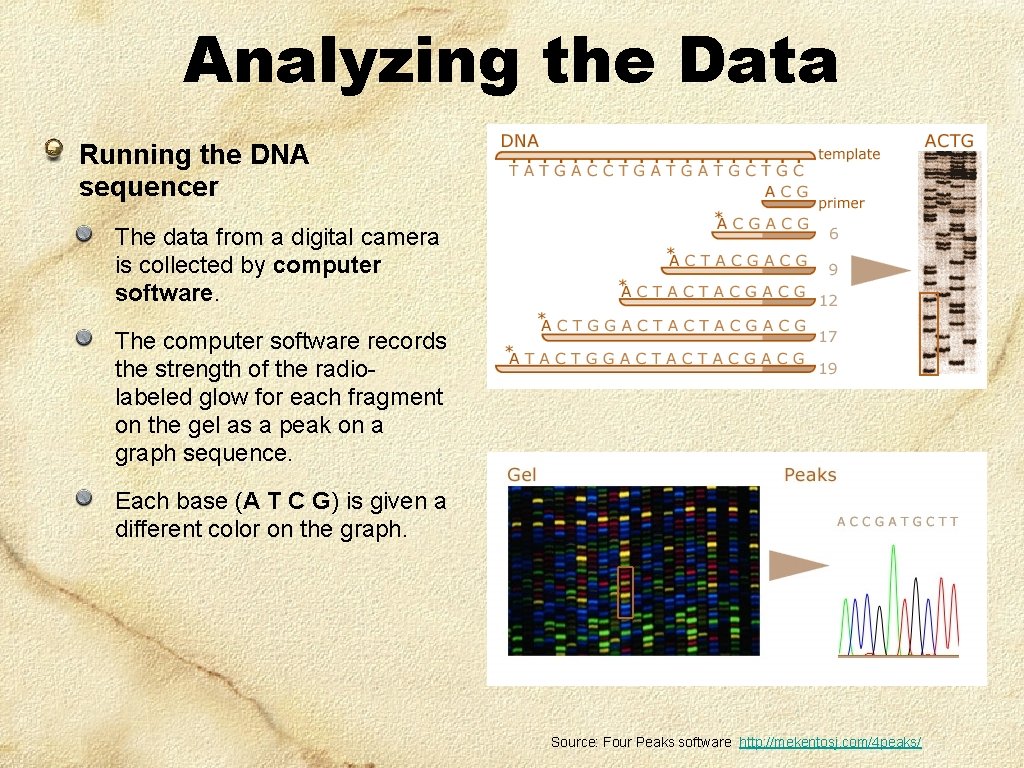
Analyzing the Data Running the DNA sequencer The data from a digital camera is collected by computer software. The computer software records the strength of the radiolabeled glow for each fragment on the gel as a peak on a graph sequence. Each base (A T C G) is given a different color on the graph. Source: Four Peaks software http: //mekentosj. com/4 peaks/

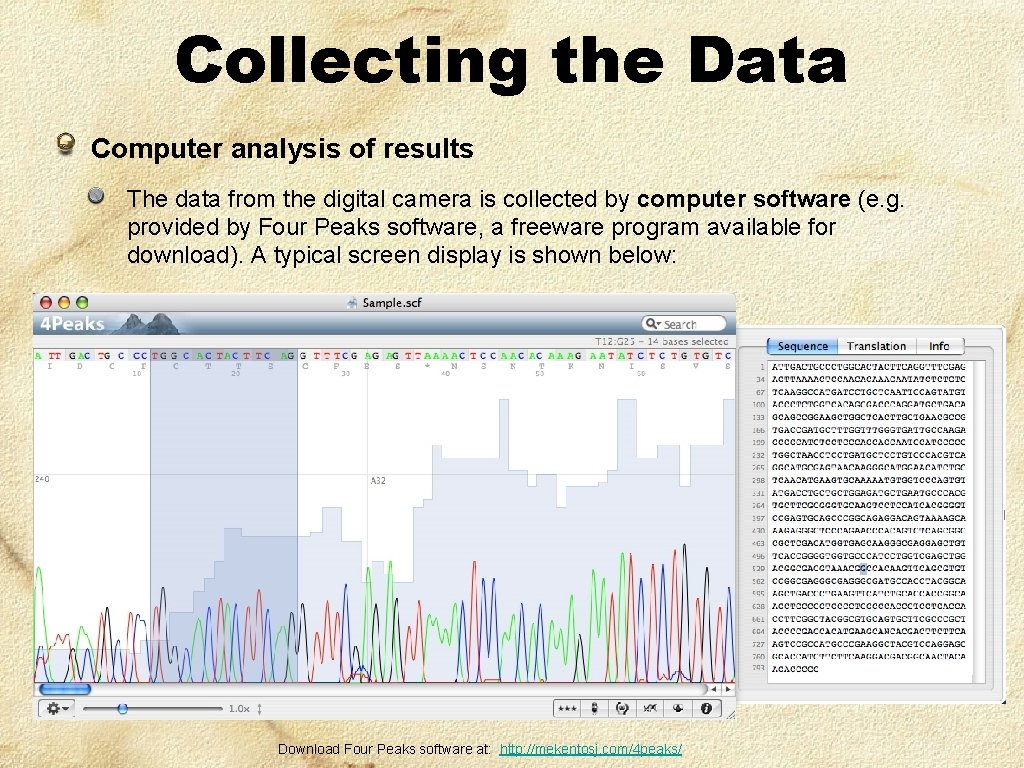
Collecting the Data Computer analysis of results The data from the digital camera is collected by computer software (e. g. provided by Four Peaks software, a freeware program available for download). A typical screen display is shown below: Download Four Peaks software at: http: //mekentosj. com/4 peaks/

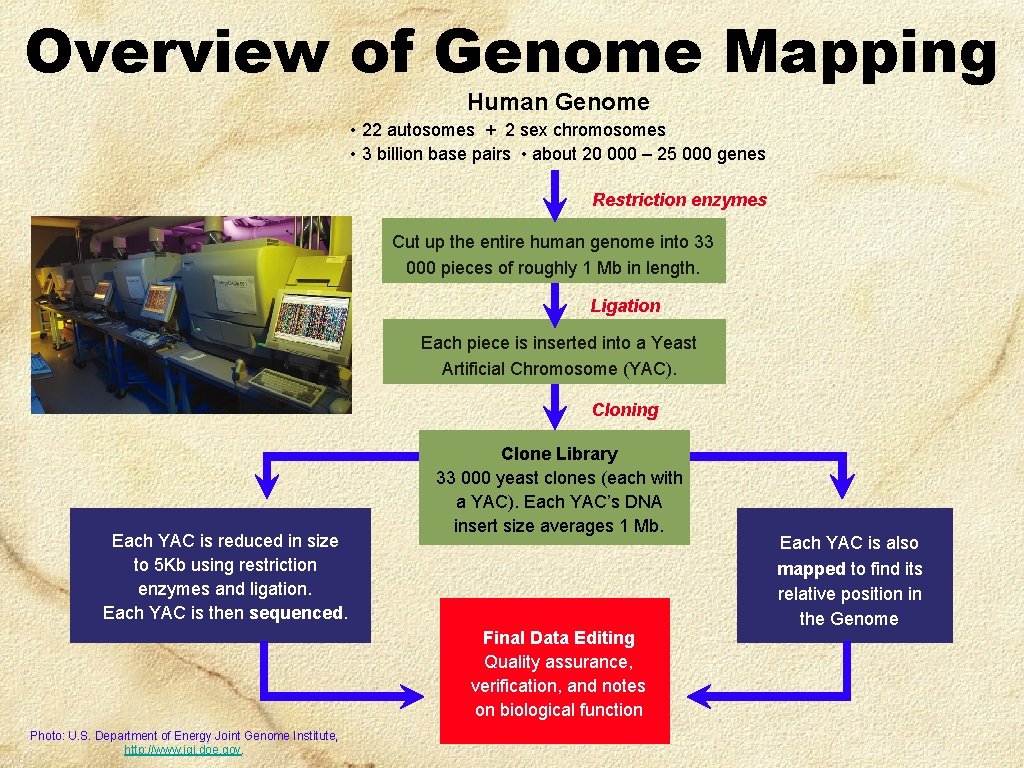
Overview of Genome Mapping Human Genome • 22 autosomes + 2 sex chromosomes • 3 billion base pairs • about 20 000 – 25 000 genes Restriction enzymes Cut up the entire human genome into 33 000 pieces of roughly 1 Mb in length. Ligation Each piece is inserted into a Yeast Artificial Chromosome (YAC). Cloning Each YAC is reduced in size to 5 Kb using restriction enzymes and ligation. Each YAC is then sequenced. Clone Library 33 000 yeast clones (each with a YAC). Each YAC’s DNA insert size averages 1 Mb. Final Data Editing Quality assurance, verification, and notes on biological function Photo: U. S. Department of Energy Joint Genome Institute, http: //www. jgi. doe. gov. Each YAC is also mapped to find its relative position in the Genome

Genome Projects Over one hundred bacterial and viral genomes, as well as a number of larger genomes have already been sequenced, including: rice, honeybee, nematode worm, African clawed frog, pufferfish, zebra fish, cow, dog, and rat Genomes that are high priority for DNA sequencing include the sea urchin, kangaroo, pig, cat, baboon, silkworm, rhesus macaque monkey, and turkey. Model organisms offer a cost-effective way to follow the inheritance of genes (which are very similar to human genes) through many generations in a relatively short time. Genome sizes and the number of genes per genome vary, and are not necessarily correlated with the size and structural complexity of the organism itself.

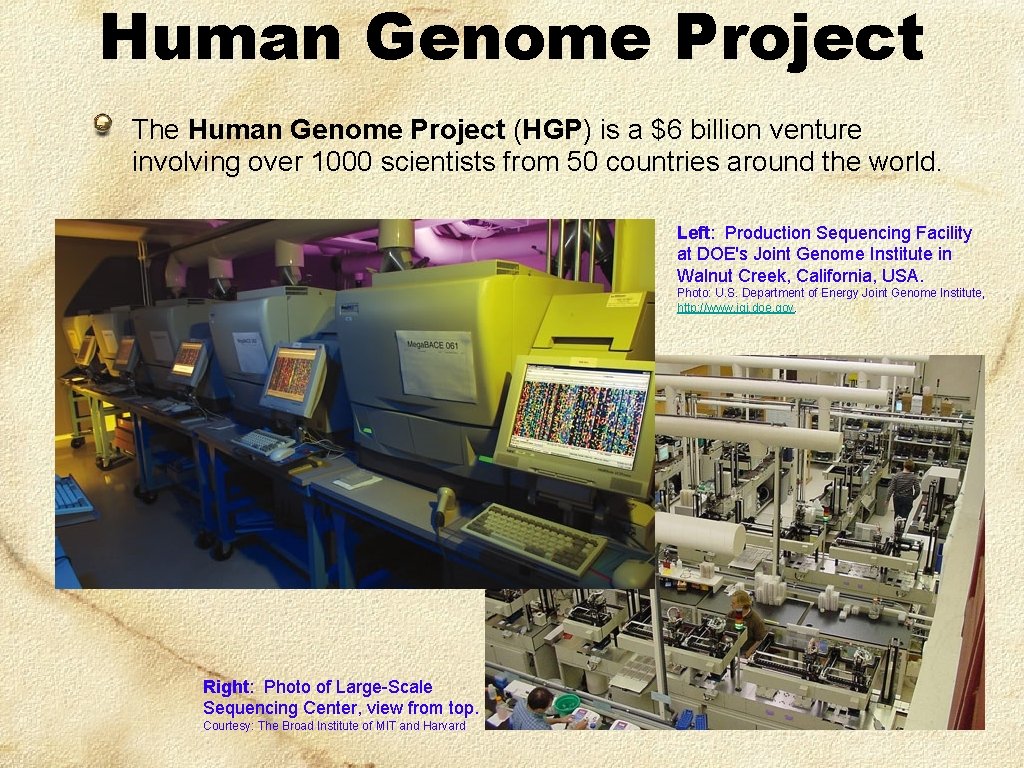
Human Genome Project The Human Genome Project (HGP) is a $6 billion venture involving over 1000 scientists from 50 countries around the world. Left: Production Sequencing Facility at DOE's Joint Genome Institute in Walnut Creek, California, USA. Photo: U. S. Department of Energy Joint Genome Institute, http: //www. jgi. doe. gov. Right: Photo of Large-Scale Sequencing Center, view from top. Courtesy: The Broad Institute of MIT and Harvard

HGP Goals Project goals were to: Identify all the approximately 20 000 -25 000 protein-coding genes in human DNA. Determine the sequences of the 3 billion chemical base pairs that make up human DNA. Store this information in databases. Improve tools for data analysis. Transfer related technologies to the private sector. Address the ethical, legal, and social issues that may arise from the project.

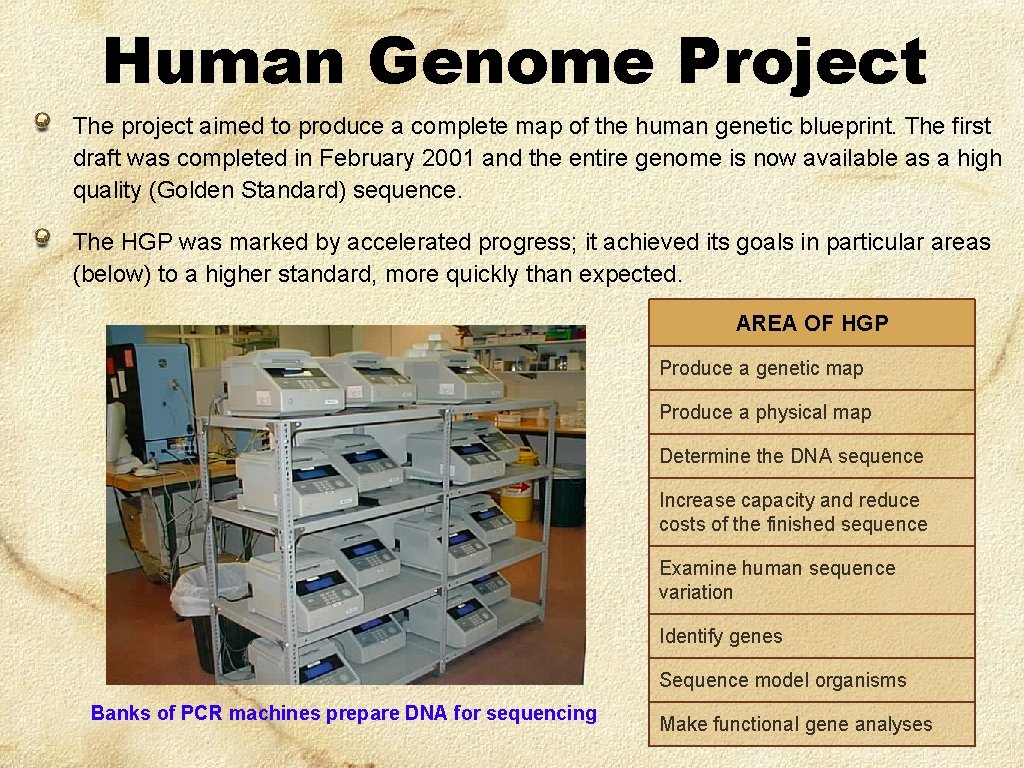
Human Genome Project The project aimed to produce a complete map of the human genetic blueprint. The first draft was completed in February 2001 and the entire genome is now available as a high quality (Golden Standard) sequence. The HGP was marked by accelerated progress; it achieved its goals in particular areas (below) to a higher standard, more quickly than expected. AREA OF HGP Produce a genetic map Produce a physical map Determine the DNA sequence Increase capacity and reduce costs of the finished sequence Examine human sequence variation Identify genes Sequence model organisms Banks of PCR machines prepare DNA for sequencing Make functional gene analyses

Benefits of the HGP It is hoped that mapping the human genome will lead to a complete understanding of the genetic basis of humans as well as the source of nearly 4000 known genetic disorders. Genes already identified as leading to disease include: Huntington’s disease, cystic fibrosis, the most common form of skin cancer, and breast cancer. Mapping and sequencing of all human genes will allow: Development of a new therapeutic drugs. Production of virtually any human protein. Design of new molecules to specifically block metabolic pathways that lead to disease. Development of gene therapy procedures for all genetically determined diseases.

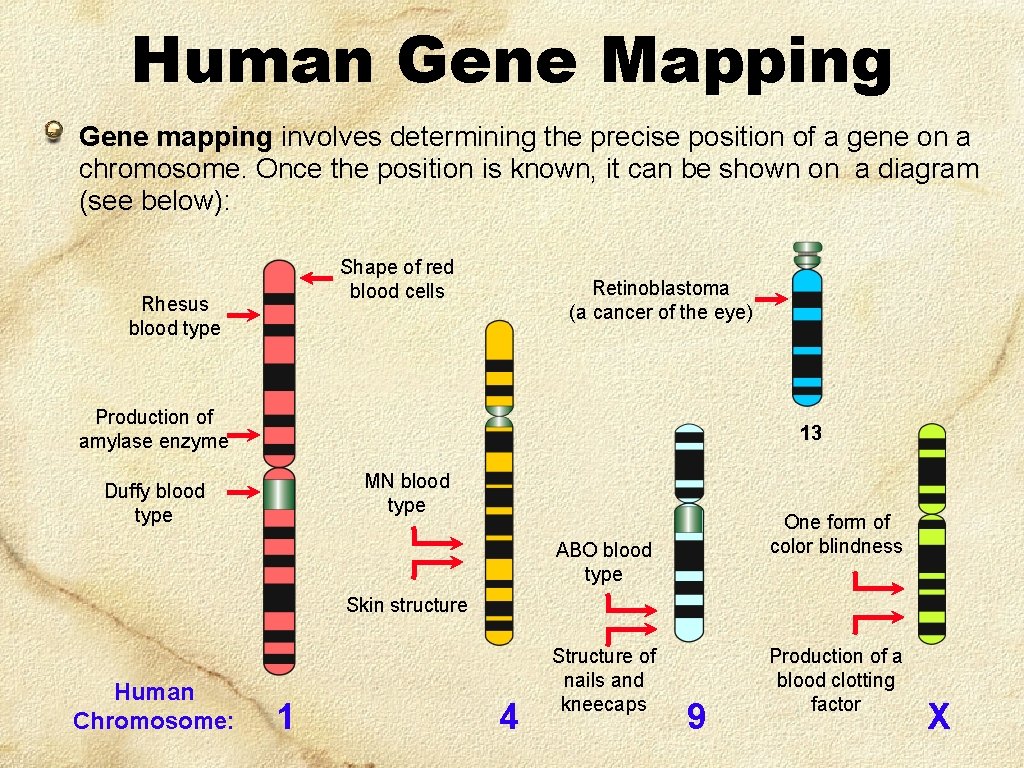
Human Gene Mapping Gene mapping involves determining the precise position of a gene on a chromosome. Once the position is known, it can be shown on a diagram (see below): Shape of red blood cells Rhesus blood type Retinoblastoma (a cancer of the eye) Production of amylase enzyme 13 MN blood type Duffy blood type One form of color blindness ABO blood type Skin structure Human Chromosome: 1 4 Structure of nails and kneecaps 9 Production of a blood clotting factor X

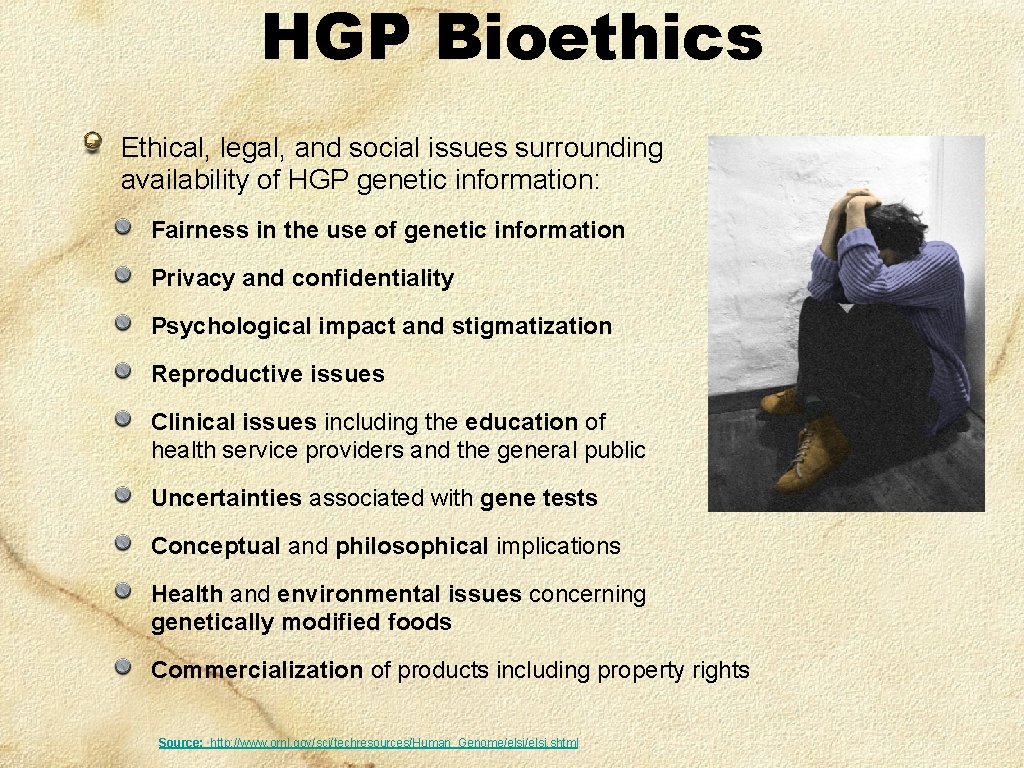
HGP Bioethics Ethical, legal, and social issues surrounding availability of HGP genetic information: Fairness in the use of genetic information Privacy and confidentiality Psychological impact and stigmatization Reproductive issues Clinical issues including the education of health service providers and the general public Uncertainties associated with gene tests Conceptual and philosophical implications Health and environmental issues concerning genetically modified foods Commercialization of products including property rights Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml

HGP Bioethics Societal concerns arising from the HGP: Fairness in the use of genetic information by insurers, employers, courts, schools, adoption agencies, and the military, among others. Who should have access to personal genetic information, and how will it be used? Privacy and confidentiality of genetic information. Who owns and controls genetic information? Psychological impact and stigmatization due to an individual's genetic differences. How does personal genetic information affect an individual and society's perceptions of that individual? How does genomic information affect members of minority communities? Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml

HGP Bioethics Societal concerns arising from the HGP: Reproductive issues including adequate informed consent for complex and potentially controversial procedures, use of genetic information in reproductive decision making, and reproductive rights. Do healthcare personnel properly counsel parents about the risks and limitations of genetic technology? How reliable and useful is fetal genetic testing? What are the larger societal issues raised by new reproductive technologies? Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml

HGP Bioethics Societal concerns arising from the HGP: Clinical issues including the education of doctors and other health service providers, patients, and the general public in genetic capabilities, scientific limitations, and social risks. Implementation of standards and quality-control measures in testing procedures. How will genetic tests be evaluated and regulated for accuracy, reliability, and utility? How do we prepare healthcare professionals for the new genetics? How do we prepare the public to make informed choices? How do we as a society balance current scientific limitations and social risk with longterm benefits? Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml

HGP Bioethics Societal concerns arising from the HGP: Uncertainties associated with gene tests for susceptibilities and complex conditions (e. g. heart disease) linked to multiple genes and gene-environment interactions. Should testing be performed when no treatment is available? Should parents have the right to have their minor children tested for adultonset diseases? Are genetic tests reliable and interpretable by the medical community? Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml

HGP Bioethics Societal concerns arising from the HGP: Conceptual and philosophical implications regarding human responsibility, free will vs genetic determinism, and concepts of health and disease. Do people's genes make them behave in a particular way? Can people always control their behavior? What is considered acceptable diversity? Where is the line between medical treatment and enhancement? Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml

HGP Bioethics Societal concerns arising from the HGP: Health and environmental issues concerning genetically modified foods (GM) and microbes. Are GM foods and other products safe to humans and the environment? How will these technologies affect developing nations' dependence on the West? Commercialization of products including property rights (patents, copyrights, and trade secrets) and accessibility of data and materials. Who owns genes and other pieces of DNA? Will patenting DNA sequences limit their accessibility and development into useful products? ©® Source: http: //www. ornl. gov/sci/techresources/Human_Genome/elsi. shtml