What is Equilibrium 1 What are characteristics of


What is Equilibrium? 1. What are characteristics of a system at equilibrium? 2. What is equilibrium?

How can we change equilibrium? 1. What are the ways to make collisions occur in a reaction? (There are 5…) 2. How is a chemical reaction like a relationship? 3. What is a common theme from the first video?

How have you experienced equilibrium? 1. What is the Haber process? 2. How is the Haber process related to equilibrium? 3. What are common themes of equilibrium?

Analogies (Change order? ) 1. How is a relationship like equilibrium? Balance in the relationship 2. How does market equilibrium apply? Supply = Demand 3. How is your daily routine like equilibrium? Balance in your day
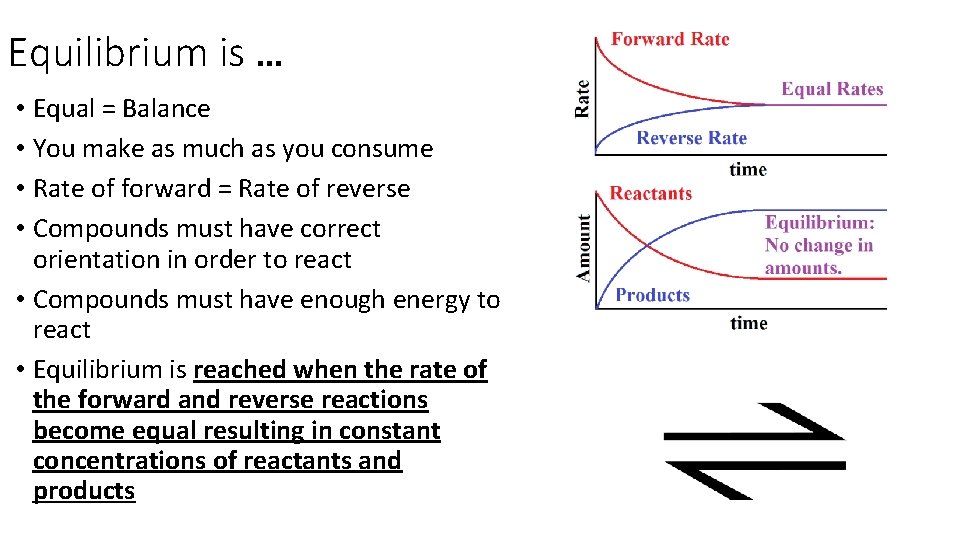
Equilibrium is … • Equal = Balance • You make as much as you consume • Rate of forward = Rate of reverse • Compounds must have correct orientation in order to react • Compounds must have enough energy to react • Equilibrium is reached when the rate of the forward and reverse reactions become equal resulting in constant concentrations of reactants and products
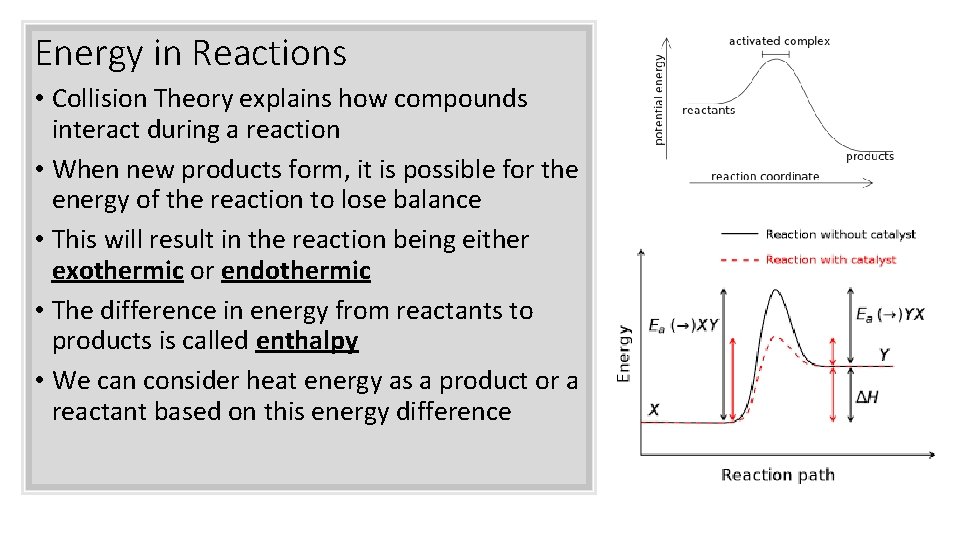
Energy in Reactions • Collision Theory explains how compounds interact during a reaction • When new products form, it is possible for the energy of the reaction to lose balance • This will result in the reaction being either exothermic or endothermic • The difference in energy from reactants to products is called enthalpy • We can consider heat energy as a product or a reactant based on this energy difference
Le Chatlelier’s Principle 1. Explains how systems at equilibrium shift in response to stress 2. 3 stresses include: Concentration, Temperature, and Pressure 3. Shifts are predictable based on Le Chatlelier's Principle
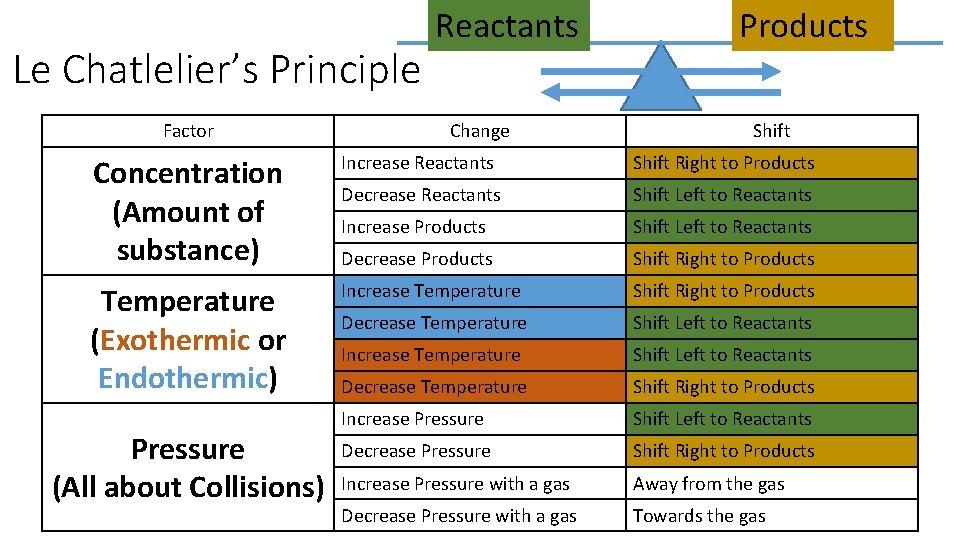
Le Chatlelier’s Principle Factor Reactants Change Products Shift Concentration (Amount of substance) Increase Reactants Shift Right to Products Decrease Reactants Shift Left to Reactants Increase Products Shift Left to Reactants Decrease Products Shift Right to Products Temperature (Exothermic or Endothermic) Increase Temperature Shift Right to Products Decrease Temperature Shift Left to Reactants Increase Temperature Shift Left to Reactants Decrease Temperature Shift Right to Products Increase Pressure Shift Left to Reactants Decrease Pressure Shift Right to Products Increase Pressure with a gas Away from the gas Decrease Pressure with a gas Towards the gas Pressure (All about Collisions)
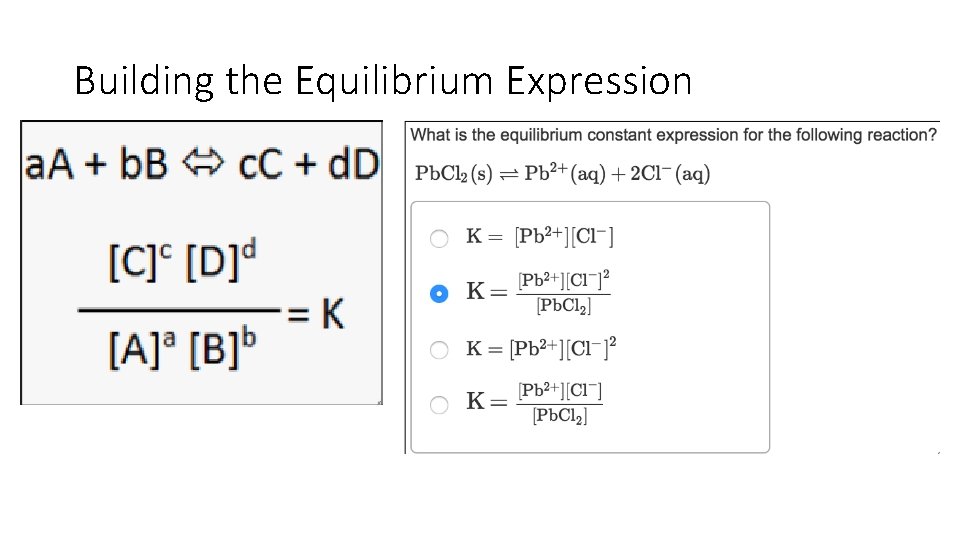
Building the Equilibrium Expression
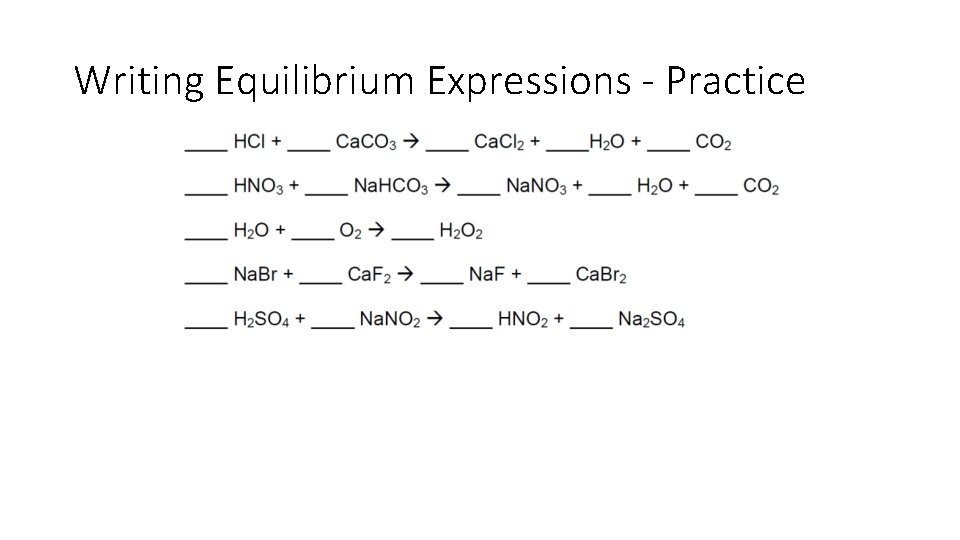
Writing Equilibrium Expressions - Practice

Equilibrium in Action Our Blood Hb + 4 O 2 ↔ Hb(O 2)4 High altitude? This is how blood doping occurs! Swimming Pools Na. Cl. O + H 2 O ↔ Na+ + OH- + HCl. O Hypochlorous Acid is a bactericidal agent formed from Hypochlorite Salt Decaffeinated Coffee
- Slides: 12