What is electronic spectroscopy Absorption of radiation leading

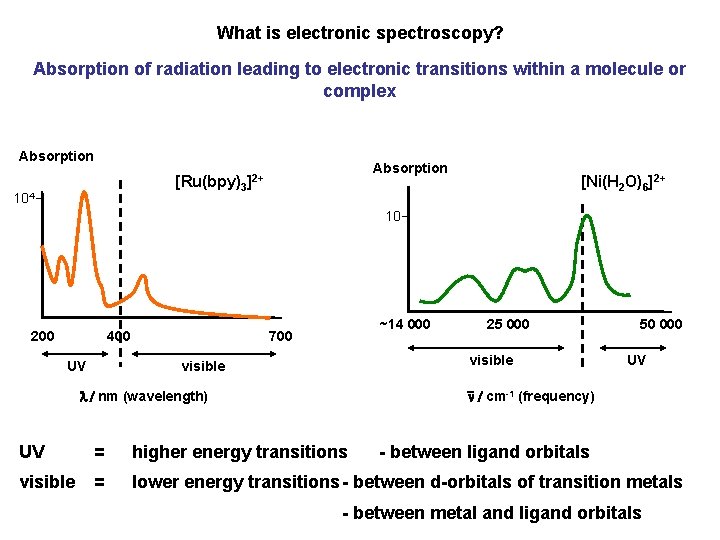
What is electronic spectroscopy? Absorption of radiation leading to electronic transitions within a molecule or complex Absorption [Ru(bpy)3]2+ 104 [Ni(H 2 O)6]2+ 10 200 400 UV ~14 000 700 25 000 visible 50 000 UV n / cm-1 (frequency) l / nm (wavelength) UV = higher energy transitions - between ligand orbitals visible = lower energy transitions - between d-orbitals of transition metals - between metal and ligand orbitals
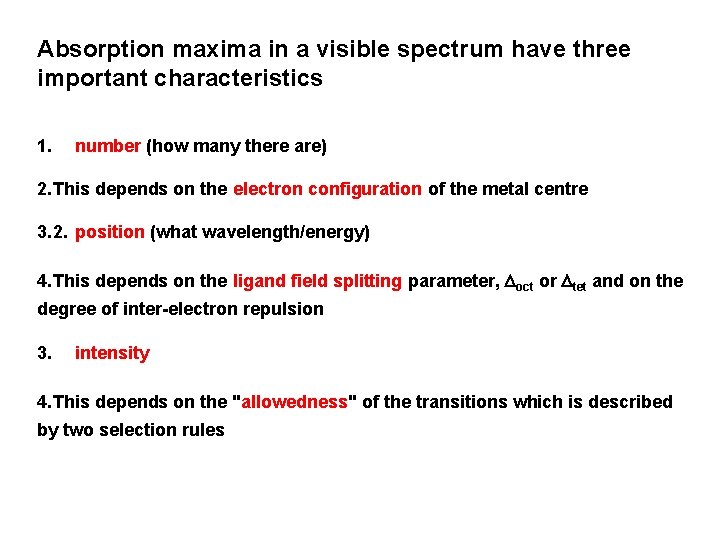
Absorption maxima in a visible spectrum have three important characteristics 1. number (how many there are) 2. This depends on the electron configuration of the metal centre 3. 2. position (what wavelength/energy) 4. This depends on the ligand field splitting parameter, Doct or Dtet and on the degree of inter-electron repulsion 3. intensity 4. This depends on the "allowedness" of the transitions which is described by two selection rules
![Absorption of light [Ti(OH 2)6]3+ = d 1 ion, octahedral complex white light 400 Absorption of light [Ti(OH 2)6]3+ = d 1 ion, octahedral complex white light 400](http://slidetodoc.com/presentation_image_h2/62099c132f6562f1b46c31ab4cabd313/image-4.jpg)
Absorption of light [Ti(OH 2)6]3+ = d 1 ion, octahedral complex white light 400 -800 nm 3+ blue: 400 -490 nm Ti yellow-green: 490 -580 nm yellow red: 580 -700 nm A This complex is has a light purple colour in solution because it absorbs green light l / nm lmax = 510 nm
![The energy of the absorption by [Ti(OH 2)6]3+ is the ligand-field splitting, Do ES The energy of the absorption by [Ti(OH 2)6]3+ is the ligand-field splitting, Do ES](http://slidetodoc.com/presentation_image_h2/62099c132f6562f1b46c31ab4cabd313/image-5.jpg)
The energy of the absorption by [Ti(OH 2)6]3+ is the ligand-field splitting, Do ES ES eg hn eg Do GS t 2 g complex in electronic Ground State (GS) [Ti(OH 2)6]3+ GS t 2 g complex in electronic excited state (ES) d-d transition lmax = 510 nm Do is 243 k. J mol-1 20 300 cm-1 An electron changes orbital; the ion changes energy state
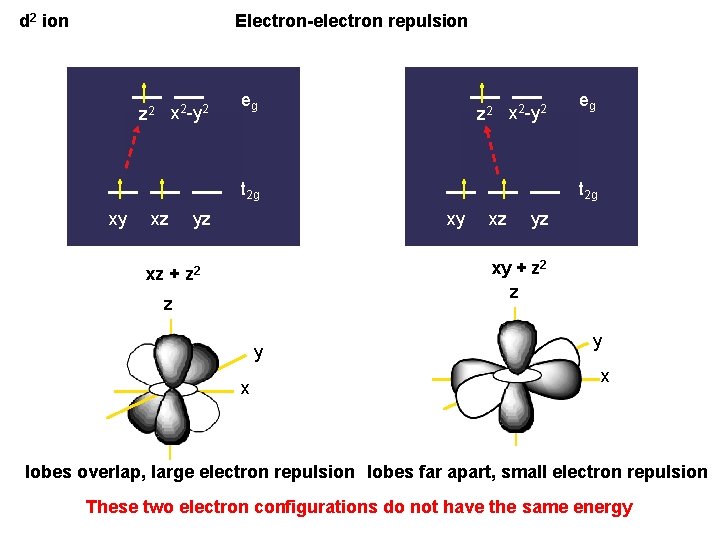
d 2 ion Electron-electron repulsion z 2 x 2 -y 2 eg z 2 x 2 -y 2 t 2 g xy xz yz eg t 2 g xy xz yz xy + z 2 z xz + z 2 z y x lobes overlap, large electron repulsion lobes far apart, small electron repulsion These two electron configurations do not have the same energy
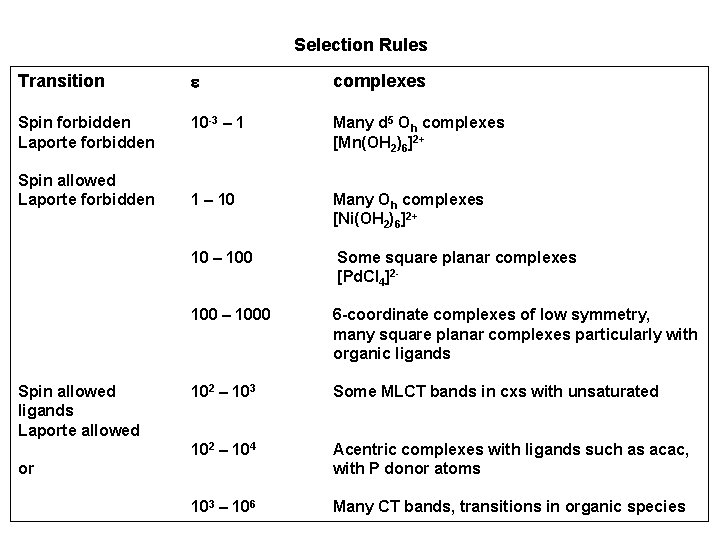
Selection Rules Transition e complexes Spin forbidden Laporte forbidden 10 -3 – 1 Many d 5 Oh complexes [Mn(OH 2)6]2+ 1 – 10 Many Oh complexes [Ni(OH 2)6]2+ 10 – 100 Some square planar complexes [Pd. Cl 4]2 - 100 – 1000 6 -coordinate complexes of low symmetry, many square planar complexes particularly with organic ligands 102 – 103 Some MLCT bands in cxs with unsaturated 102 – 104 Acentric complexes with ligands such as acac, with P donor atoms 103 – 106 Many CT bands, transitions in organic species Spin allowed Laporte forbidden Spin allowed ligands Laporte allowed or
![Tanabe-Sugano diagram for d 2 ions 10 e [V(H 2 O)6]3+: Three spin allowed Tanabe-Sugano diagram for d 2 ions 10 e [V(H 2 O)6]3+: Three spin allowed](http://slidetodoc.com/presentation_image_h2/62099c132f6562f1b46c31ab4cabd313/image-8.jpg)
Tanabe-Sugano diagram for d 2 ions 10 e [V(H 2 O)6]3+: Three spin allowed transitions 5 E/B 30 000 20 000 n 1 = 17 800 cm-1 visible n 2 = 25 700 cm-1 visible 10 000 n / cm-1 n 3 = obscured by CT transition in UV 25 700 = 1. 44 D/B 17 800 n 3 = 2. 1 n 1 = 2. 1 x 17 800 D/B = 32 n 3 = 37 000 cm-1 = 32
Magnetism

macroscopic world « traditional, classical » magnets N S
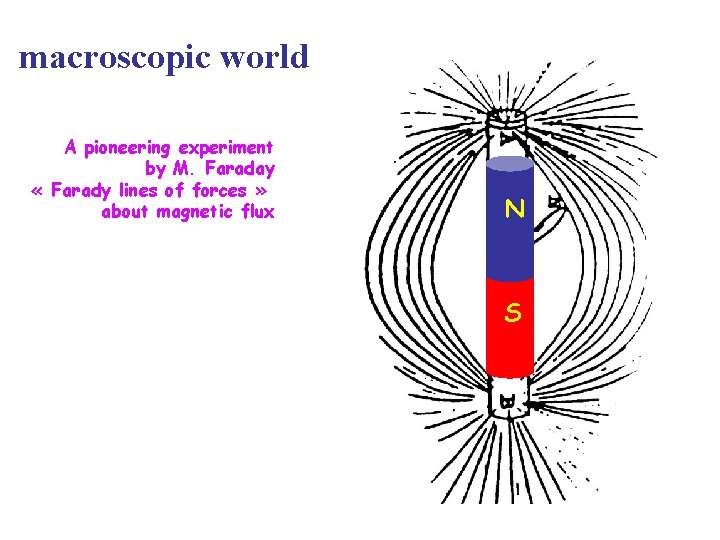
macroscopic world A pioneering experiment by M. Faraday « Farady lines of forces » about magnetic flux N S
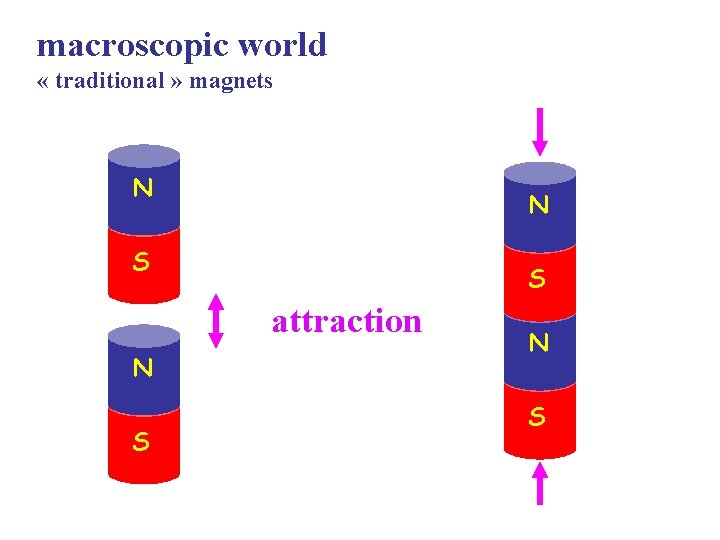
macroscopic world « traditional » magnets N N S S attraction N S
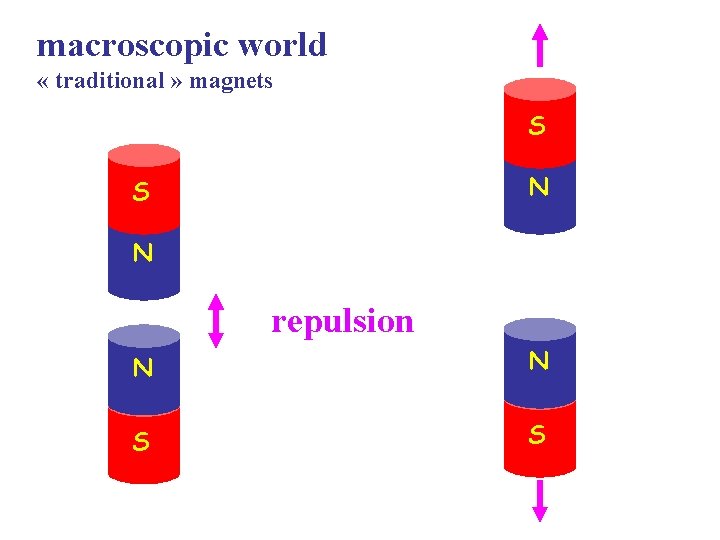
macroscopic world « traditional » magnets S N repulsion N N S S
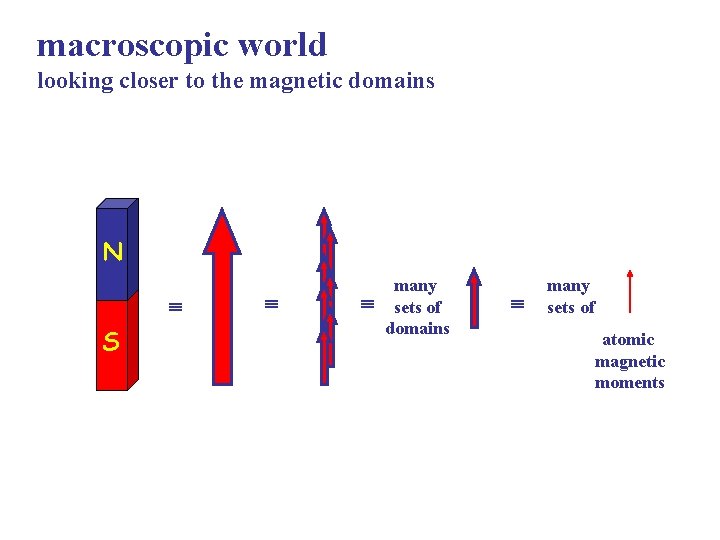
macroscopic world looking closer to the magnetic domains N S many sets of domains many sets of atomic magnetic moments
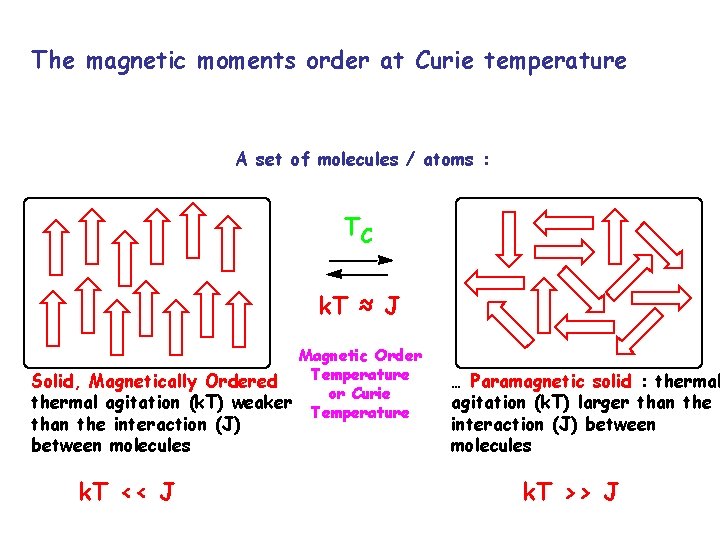
The magnetic moments order at Curie temperature A set of molecules / atoms : TC k. T ≈ J Magnetic Order Temperature Solid, Magnetically Ordered or Curie thermal agitation (k. T) weaker Temperature than the interaction (J) between molecules k. T << J … Paramagnetic solid : thermal agitation (k. T) larger than the interaction (J) between molecules k. T >> J

Magnetic Order : ferro-, antiferro- and ferri-magnetism Ferromagnetism : Magnetic moments are identical and parallel + = Ferrimagnetism (Néel) : Magnetic moments are different and anti parallel Antiferromagnetism : Magnetic moments are identical and anti parallel + = 0 + =
Origin of Magnetism … the electron I am an electron • rest mass me, • charge e-, • magnetic moment µB everything, tiny, elementary
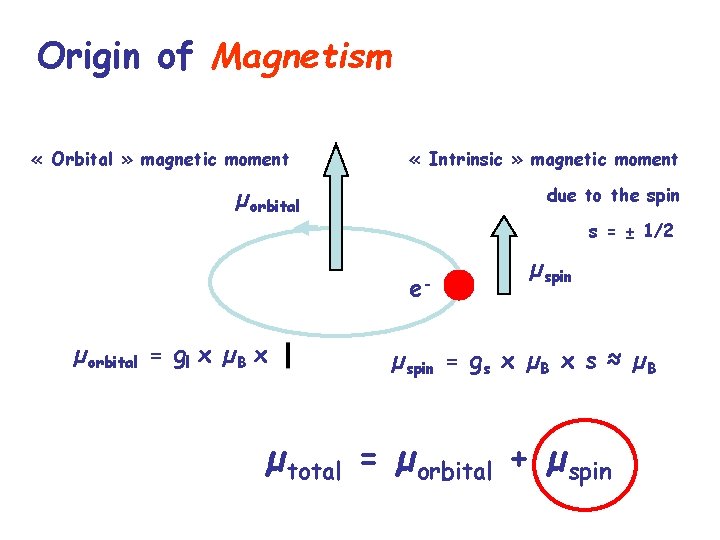
Origin of Magnetism « Orbital » magnetic moment « Intrinsic » magnetic moment µorbital due to the spin s = ± 1/2 eµorbital = gl x µB x µspin = gs x µB x s ≈ µB µtotal = µorbital + µspin

Dirac Equation The Principles of Quantum Mechanics, 1930 1905 Nobel Prize 1933 1928 http: //www-history. mcs. st-and. ac. uk/history/Pict. Display/Dirac. html
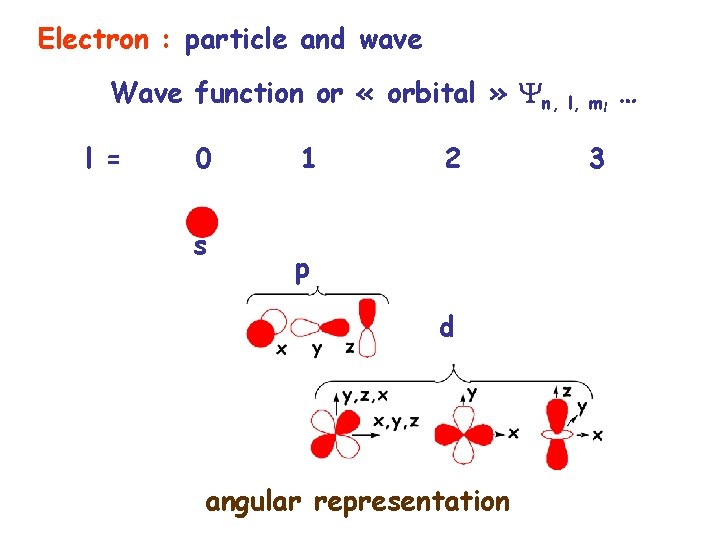
Electron : particle and wave Wave function or « orbital » n, l, ml … l = 0 s 1 2 p d angular representation 3
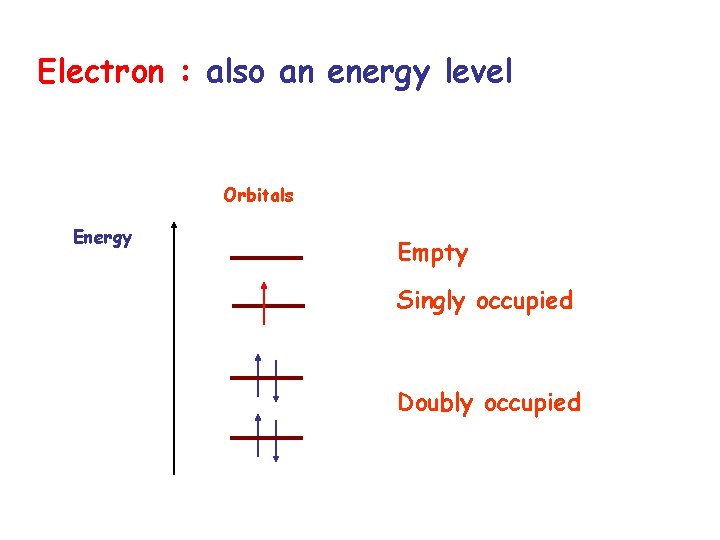
Electron : also an energy level Orbitals Energy Empty Singly occupied Doubly occupied
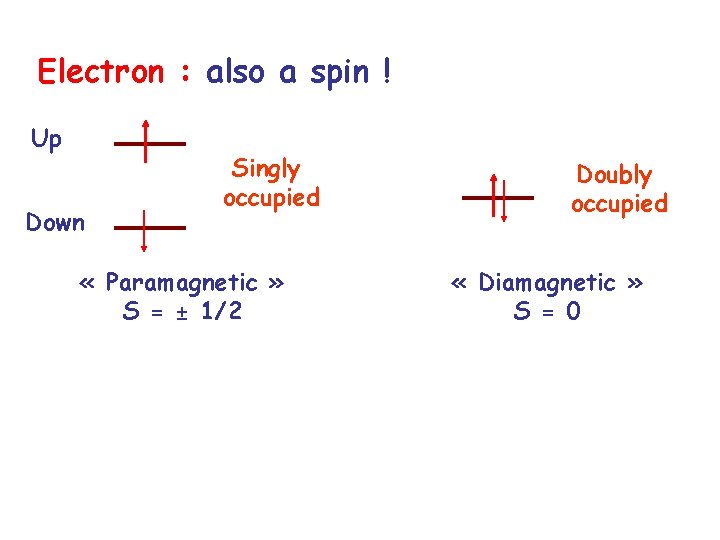
Electron : also a spin ! Up Down Singly occupied « Paramagnetic » S = ± 1/2 Doubly occupied « Diamagnetic » S = 0
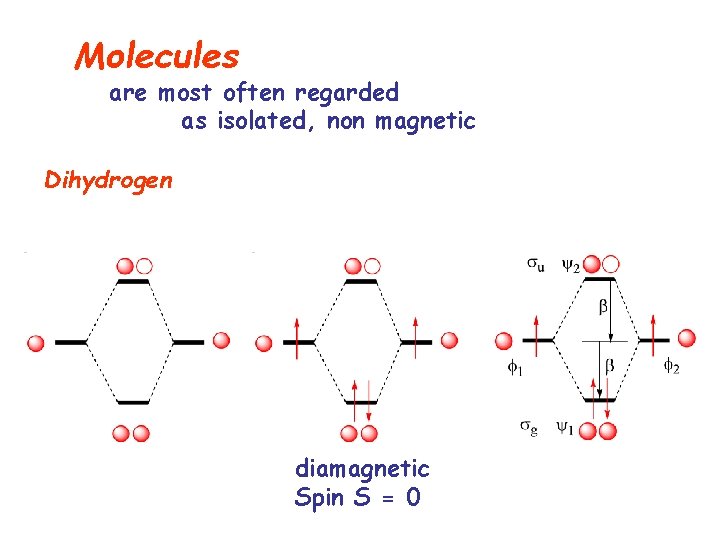
Molecules are most often regarded as isolated, non magnetic Dihydrogen diamagnetic Spin S = 0

the dioxygen that we continuously breath is a magnetic molecule orthogonal π molecular orbitals paramagnetic, spin S =1 Two of its electrons have parallel magnetic moments that shapes aerobic life and allows our existence as human beings

Transition Elements
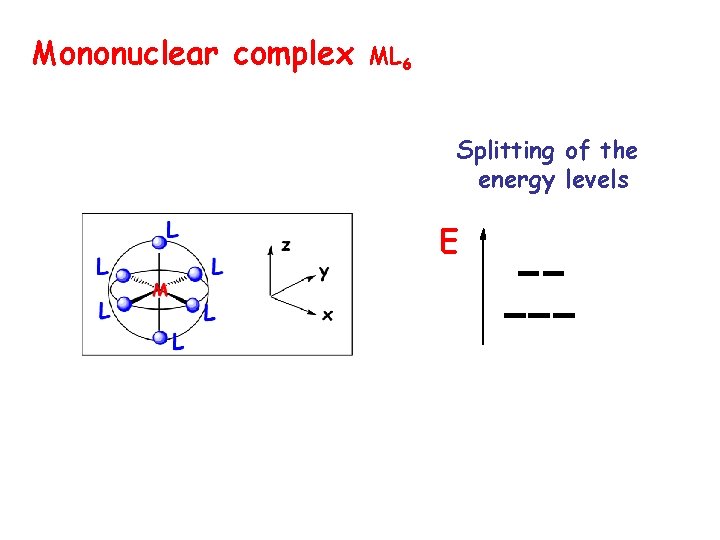
Mononuclear complex ML 6 Splitting of the energy levels E
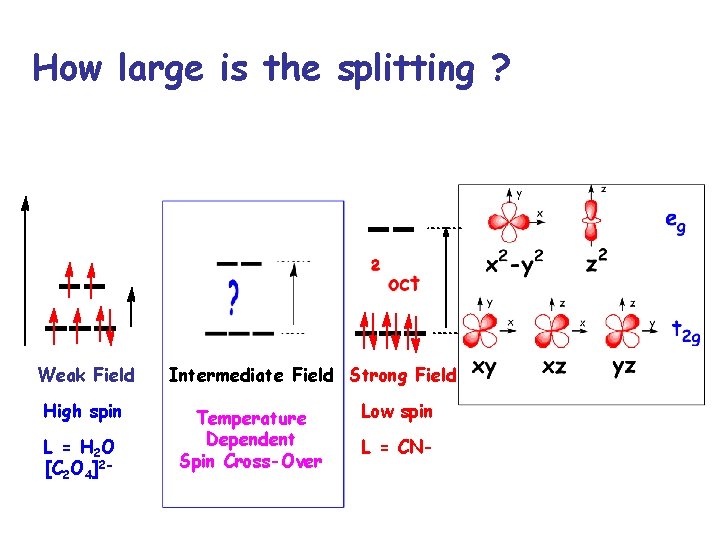
How large is the splitting ? Weak Field High spin L = H 2 O [C 2 O 4]2 - Intermediate Field Strong Field Temperature Dependent Spin Cross-Over Low spin L = CN-
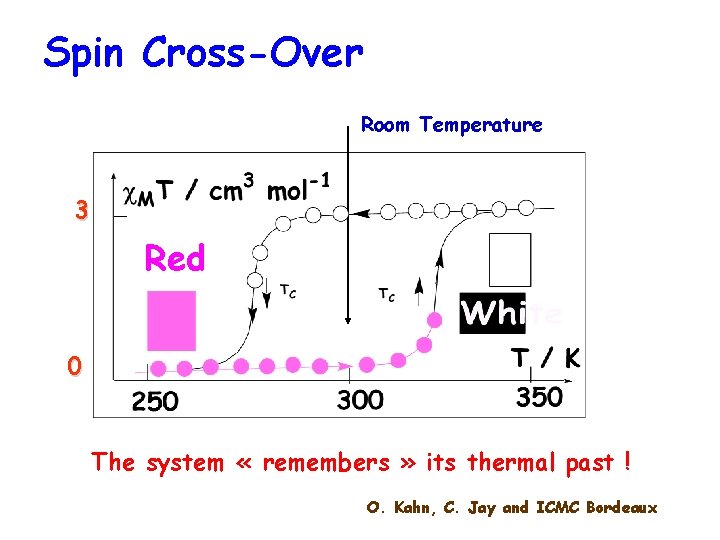
Spin Cross-Over Room Temperature 3 Red 0 The system « remembers » its thermal past ! O. Kahn, C. Jay and ICMC Bordeaux
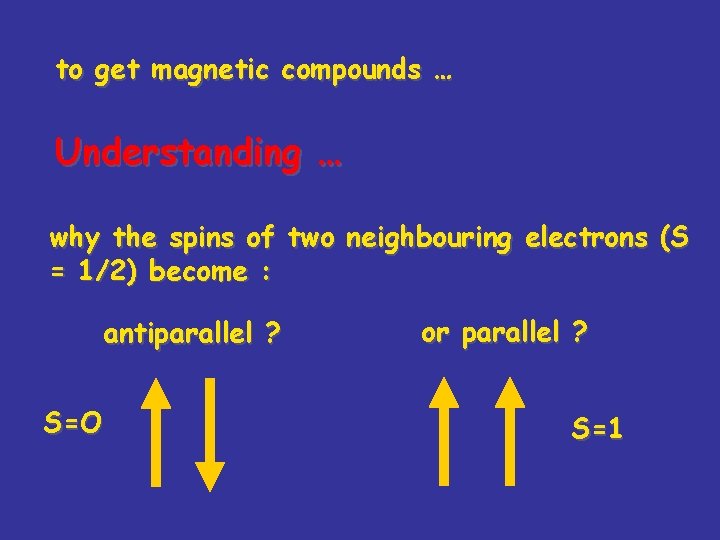
to get magnetic compounds … Understanding … why the spins of two neighbouring electrons (S = 1/2) become : antiparallel ? S=O or parallel ? S=1
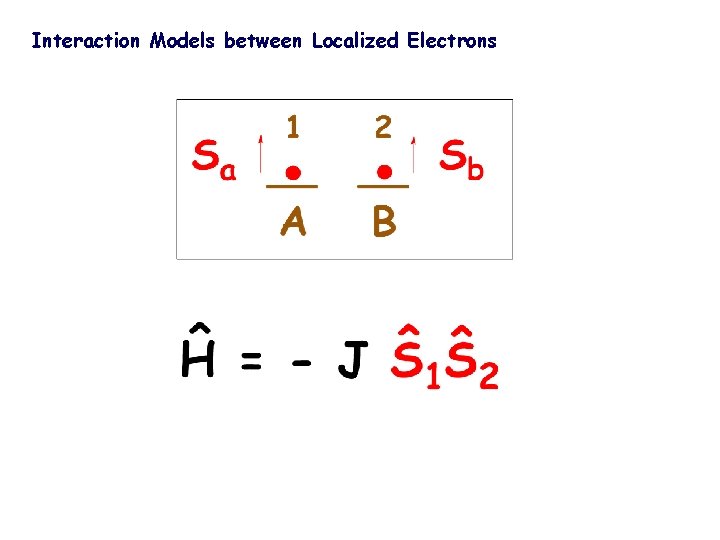
Interaction Models between Localized Electrons
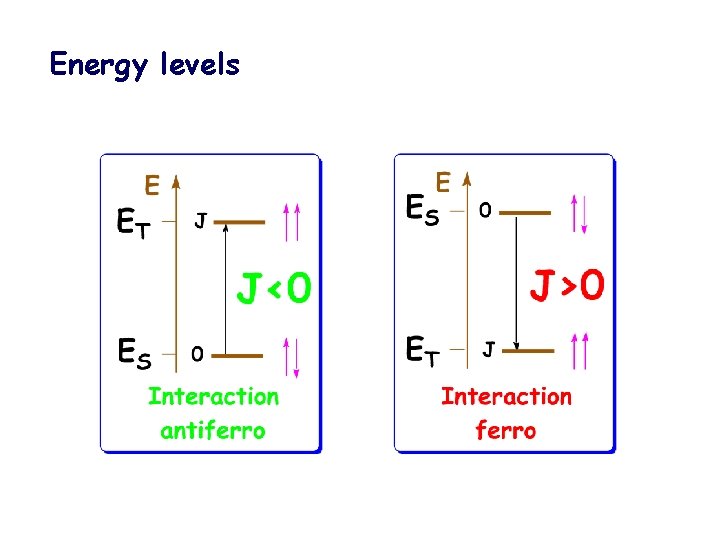
Energy levels
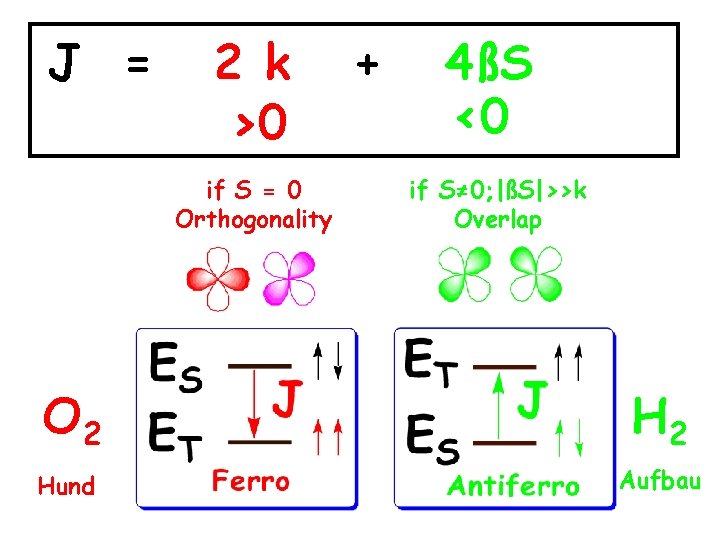
J = 2 k >0 if S = 0 Orthogonality + 4ßS <0 if S≠ 0; |ßS|>>k Overlap O 2 Hund Aufbau
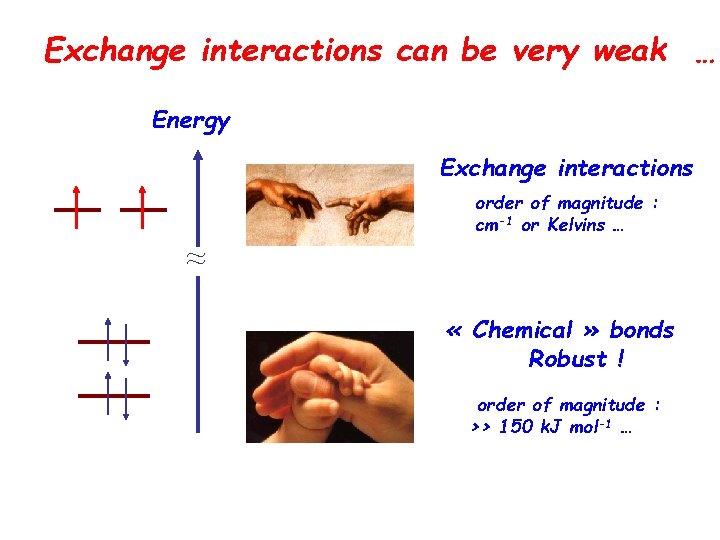
Exchange interactions can be very weak … Energy Exchange interactions order of magnitude : cm-1 or Kelvins … ≈ « Chemical » bonds Robust ! order of magnitude : >> 150 k. J mol-1 …
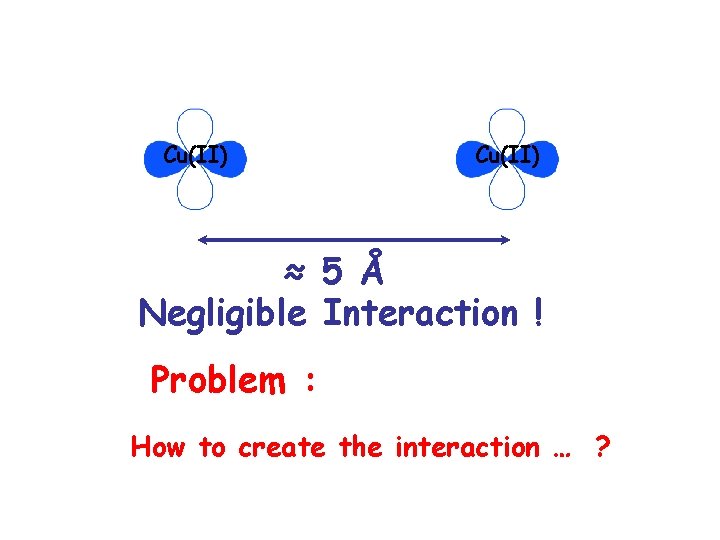
Cu(II) ≈ 5 Å Negligible Interaction ! Problem : How to create the interaction … ?
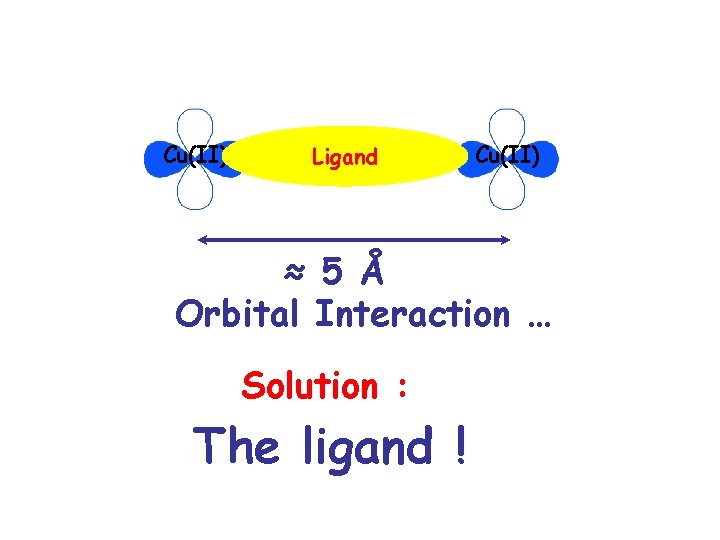
Cu(II) Ligand Cu(II) ≈ 5 Å Orbital Interaction … Solution : The ligand !
A Ligand B Examples with the ligand • Cyanide
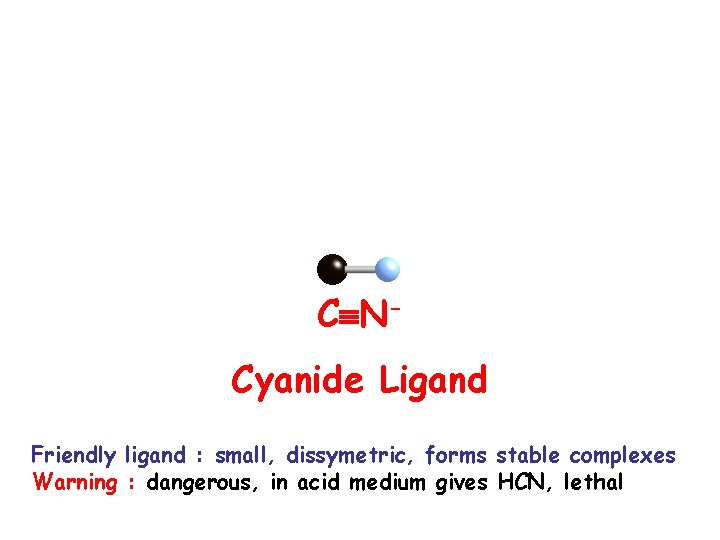
C NCyanide Ligand Friendly ligand : small, dissymetric, forms stable complexes Warning : dangerous, in acid medium gives HCN, lethal
Dinuclear µ-cyano homometallic complexes
![“Models” Compounds Cu(II)-CN-Cu(II) J/cm-1 Compounds exp [Cu 2(tren)2 CN]3+ -160 [Cu 2(tmpa)2 CN]3+ -100 “Models” Compounds Cu(II)-CN-Cu(II) J/cm-1 Compounds exp [Cu 2(tren)2 CN]3+ -160 [Cu 2(tmpa)2 CN]3+ -100](http://slidetodoc.com/presentation_image_h2/62099c132f6562f1b46c31ab4cabd313/image-39.jpg)
“Models” Compounds Cu(II)-CN-Cu(II) J/cm-1 Compounds exp [Cu 2(tren)2 CN]3+ -160 [Cu 2(tmpa)2 CN]3+ -100 Overlap : antiferromatic coupling … Rodríguez-Fortea et al. Inorg. Chem. 2001, 40, 5868
- Slides: 39