What is Dry Ice Who Invented This Stuff








- Slides: 9

What is Dry Ice? Who Invented This Stuff? CAUTION: Contents Under Pressure! Dry Ice Inquiry Based Learning, Technology, & Research Project By Breezie Bitter About the Author Sources

What is Dry Ice? Dry ice is simply one thing: Solid Carbon Dioxide (aka CO 2) CO 2 is what we exhale when we breath. It is also what helps plants grow. BUT when it is put into solid form, it is EXTREMELY and becomes what know as “dry Want COLD to know how it’s made? Clickwe HERE! ice”. Click here to see what dry ice is used for!

Cool Stuff Dry Ice Is Used For! 1. 2. 3. 4. 5. 6. 7. Making carbonated drinks. (Like Root Beer!) To make ice cream. Preserve food (keep it fresh). Prevents insects from getting into grain containers. Fog effects in movies, plays, and even haunted houses! Can freeze off warts. (Ouch!) Used to bait and trap mosquitoes and insects. (They love CO 2!) 8. Blasted through an air compressor to clean machinery. 9. Helps plumbers stop water flow in pipes so they can fix things. 10. Used in laboratories to experiment with cold chemical reactions.

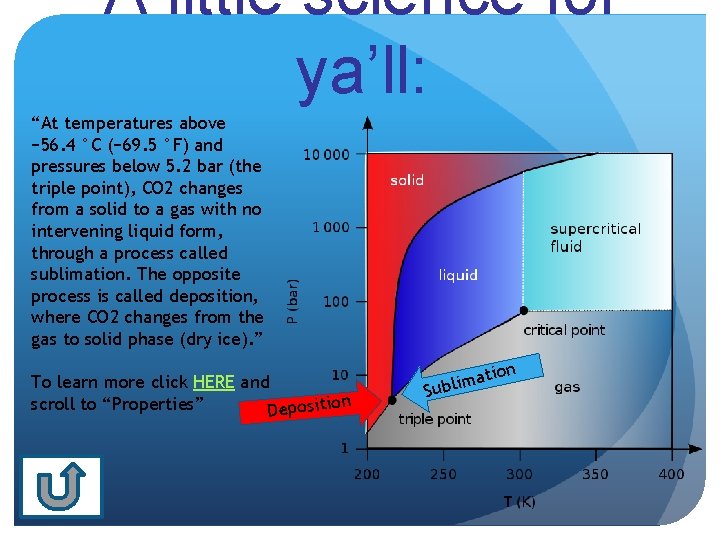
A little science for ya’ll: “At temperatures above − 56. 4 °C (− 69. 5 °F) and pressures below 5. 2 bar (the triple point), CO 2 changes from a solid to a gas with no intervening liquid form, through a process called sublimation. The opposite process is called deposition, where CO 2 changes from the gas to solid phase (dry ice). ” To learn more click HERE and n scroll to “Properties” Depositio S on ati m i l ub

Who Invented Dry Ice? Dry ice was observed in 1834 by a French chemist named Charles Thilorier. He opened a container of liquid carbon dioxide and noticed most of the liquid evaporated quickly. He also saw that there was some CO 2 left in the container, but it was solid! He had discovered dry ice. In 1924 Thomas B. Slate started figuring out ways to use dry ice and eventually patent it and sold it. Charles Thilorier

Inquiry Experiment: Will adding more dry ice to a dry ice bomb cause the bomb to explode at a faster rate? Check out the T-Chart below and then Click HERE to see what happens! Test Control • Amount of Dry Ice (oz. ) • No Dry Ice Constants • Water Level (8 oz. ) • Bottle Type/Size Hypothesis: More dry ice will make the bomb explode at a quicker rate. Click HERE for the experiment conclusion.

Results/Conclusion According to our results: 1 ½ oz. Dry Ice+ Water + Bottle= 26 second explosion rate 2 ½ oz. Dry Ice+ Water + Bottle= 26 second explosion rate Fact: 1 lb of dry ice= 8 cubic feet of gas (About enough to fill 125 2 liter bottles!) THEREFORE… 1. My experiment was not accurate and not controlled enough to show proper results. 2. More dry ice (SHOULD)= Bigger/faster explosion

About the Author: Breezie Bitter Hello! I hope you enjoyed learning about dry ice. It is such an exciting invention! I love science and to experiment with new things. I also love to teach and to learn. Outside of school I like to spend my time reading, exploring the outdoors, playing with my little boy, and spending time with friends and family.

Sources http: //en. wikipedia. org/wiki/Dry_ice http: //www. west. net/~science/co 2. htm http: //www. dryiceinfo. com/ http: //science. howstuffworks. com/innovation/question 264. htm http: //dry-ice-experiments. blogspot. com/ http: //www. weirdsciencekids. com/dryice. html