What is Dissolved Oxygen DO It IS elemental








![Air Saturation in water [25 C, 1 ATM]: 8. 24 mg O 2/L [8. Air Saturation in water [25 C, 1 ATM]: 8. 24 mg O 2/L [8.](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-10.jpg)


![Electrochemical Measurement of Dissolved Oxygen [DO] Electrochemical Measurement of Dissolved Oxygen [DO]](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-13.jpg)





![Dissolved Oxygen [DO] Equilibrium Sensor Dissolved Oxygen [DO] Equilibrium Sensor](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-19.jpg)








![…and, uniquely for the equilibrium sensor, oxygen is generated at the ANODE [positive electrode] …and, uniquely for the equilibrium sensor, oxygen is generated at the ANODE [positive electrode]](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-28.jpg)






























- Slides: 58

What is Dissolved Oxygen (DO) ?

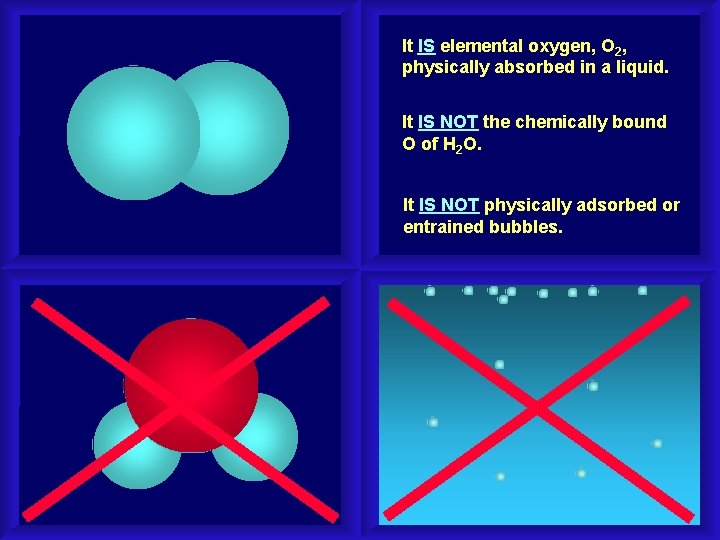
It IS elemental oxygen, O 2, physically absorbed in a liquid. It IS NOT the chemically bound O of H 2 O. It IS NOT physically adsorbed or entrained bubbles.

What is Dissolved Oxygen? Dissolved Oxygen - the amount of O 2 gas dissolved in a sample of liquid water. Units of measure: • • ppb ppm % saturation mg/liter( ppm(

How does DO get there? Chemical, Electrochemical or Biochemical processes such as Photosynthesis Exposing the liquid to air or O 2 Shaking helps

Why is Dissolved Oxygen measured? “Dissolved Oxygen is one of the most important measurements in the determination of the health of a body of water”

Who makes this measurement? In ppm Applications , DO must stay above a minimal value to support life. Fish & Shrimp Farms Wastewater Treatment Plants

In ppb Applications, DO needs to be below some maximum value to inhibit Corrosion or Food Spoilage. Semiconductor Food & Beverage Power Utilities

POWER PLANT APPLICATIONS-DISSOLVED OXYGEN-- DO Raw water Saturated Steam H-P Turbine L-P Turbine I-P Turbine Chemical Feed Make-up Water Treatment Demineralizer Condenser Drum Cooling Water Hotwell Condensate Storage Tank Super 8. 25 heater 8. 25 DO Economizer DO Blowdown 8. 25 DO Boiler Reheater 8. 25 Deaerator Condensate Polishers Feed Water H-P Heaters L-P Heaters 8. 25 DO Chemical Feed DO

How Much gets there?
![Air Saturation in water 25 C 1 ATM 8 24 mg O 2L 8 Air Saturation in water [25 C, 1 ATM]: 8. 24 mg O 2/L [8.](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-10.jpg)
Air Saturation in water [25 C, 1 ATM]: 8. 24 mg O 2/L [8. 24 ppm] Compare to O 2 in air: PV = n. RT n/V = P/RT = 1 atm/0. 082[L-atm/mole. K]x 298 K = 0. 0406 moles air x 0. 209 moles O 2/mole air x 32, 000 mg O 2/mole O 2 = 274 mg O 2/L [oxygen in air/ Oxygen in air-sat. water] = 274/8. 24 = 33. 3

Reasons to use On-line versus Off-line Measurements • • • Off-line are too slow to correct sudden upsets Off-line cannot catch momentary fluctuations With Off-line, it is easy to contaminate the sample Have to manually record other influences at time of sample collection – temperature - weather behavior – time of day - day of week Currently, no reference standard available for the ppb industry

Continuous On-line Measurement Membrane Technology - the membrane separates the internal electrolyte and electrodes from the sample, allowing only the gases to penetrate the membrane for measurement. Two Types Diffusion - based on the rate of diffusion of oxygen across a membrane. Example: Polarographic or Galvanic Equilibrium - based on the partial pressure of oxygen. sells.
![Electrochemical Measurement of Dissolved Oxygen DO Electrochemical Measurement of Dissolved Oxygen [DO]](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-13.jpg)
Electrochemical Measurement of Dissolved Oxygen [DO]

Dissolved O 2: Typical Diffusion Cell Pb Anode: 2 Pb + 2 H 2 O = Replaceable Electrolyte Inert Cathode Replaceable Membrane Diffusion Rate OC [DO] / path length 2 Pb. O + 4 H+ + 4 e- Cathode: O 2 + 4 H+ + 4 e- = 2 H 2 O Oxygen Transport

Dissolved O 2: Typical Diffusion Cell, Fouled Pb Anode Replaceable Electrolyte Inert Cathode Replaceable Membrane Oxygen Fouling Diffusion Rate OC [DO] / path length Transport. Diminished

Dissolved O 2: Typical Diffusion Cell, in Still Water Diffusion Probe: Pb Anode Fouling Dependent Flow Dependent Replaceable Electrolyte Requires: Membrane Changing Electrolyte Replenishment Electrode Treatment Inert Cathode Replaceable Membrane Stagnant [still] water Diffusion Rate OC [DO] / path length Oxygen Transport Diminished

Special Requirements of Diffusion Types Minimum Flow - since oxygen is continuously consumed at the cathode, a deficiency of oxygen may result if a steady flow of oxygen is interrupted. Therefore, a continuous minimum supply of oxygen must diffuse through the membrane for an accurate reading. No Membrane Fouling - Fouling reduces the surface area for oxygen transport, less oxygen available for reduction results in lower readings.

Advantages • • Electrolyte can be restored Lower initial cost Disadvantages • • • Anode needs to be cleaned, recharged or replaced as oxide build-up occurs (electrolyte must be replaced at this time also( Membrane is delicate - needs to be replaced periodically Probe is flow sensitive
![Dissolved Oxygen DO Equilibrium Sensor Dissolved Oxygen [DO] Equilibrium Sensor](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-19.jpg)
Dissolved Oxygen [DO] Equilibrium Sensor

Desired are. . Reliable Results Long Life Low Maintenance Low Cost of Ownership

To Achieve this. . . we invented…. The Equilibrium DO Sensor

At the end of a support probe, is the sensor consisting of. . .

A Ceramic Mandrel

encoiled by. . . inert, closely-paired bifiler electrodes

sealed in. . . a tough, permanent, gas-permeable membrane

and bathed in. . . a permanent internal electrolyte solution.

When the sensor is turned on, Oxygen immediately adjacent to the CATHODE [the negative electrode] is consumed according to - + O 2 + 4 H+ + 4 e- = 2 H 2 O
![and uniquely for the equilibrium sensor oxygen is generated at the ANODE positive electrode …and, uniquely for the equilibrium sensor, oxygen is generated at the ANODE [positive electrode]](https://slidetodoc.com/presentation_image_h/1fd3813f660a935e11a7739d3ad0356c/image-28.jpg)
…and, uniquely for the equilibrium sensor, oxygen is generated at the ANODE [positive electrode] in an amount equal to that consumed at the CATHODE. - + - 2 H 2 O = O 2 + 4 H+ + 4 e- = 2 H 2 O + The rest of the reaction balances, too.

The current supporting these reactions is directly proportional to the DO concentration. - + 2 H 2 O = O 2 + 4 H+ + 4 e- = 2 H 2 O

In Detail. . . Let’s look at just 3 windings….



Sample Sensor Oxygen, , starts uniformly distributed

Sample Sensor and then we turn the sensor ON

Sample Sensor At the Cathode, O 2 is consumed

Sample Sensor At the Anode, O 2 is generated

Sample Sensor providing high concentration at the anode

Sample Sensor and zero concentration at the cathode

But there is zero concentration gradient between the sample and the outer portion of the membrane:

DO equilibrium exists between Sensor and Sample

The oxygen flux is all within the membrane.

Sample Sensor Caveat Fouling, Still Water do not disturb equilibrium

Only when sample DO Changes does O 2 diffuse…. .

INTO or. . .

Sample Sensor OUT OF the sensor

Sample Sensor Until equilibrium is reestablished

Fouling or still water only slow reaching equilibrium

And. . . since the reactions are balanced, No chemical changes occur in the sensor and a permanent sensor is possible. - + 2 H 2 O = O 2 + 4 H+ + 4 e- = 2 H 2 O

Summary: with close-spaced inert electrodes and internal oxygen generation, the equilibrium DO sensor is. . .

Independent of fouling Independent of flow…and. . . Permanent, Requiring: NO NO NO membrane changing electrolyte replacing electrode treating For….

Reliable Results Long Life Low Maintenance Low Cost of Ownership

Honeywell DO Instrumentation 7020 Analyzer or + DL 5000 Equilibrium Probe Direct. Line®

Honeywell DO Instrumentation Model : 7022 ppm/ 7021 ppb Case : Aluminum NEMA 4 X/IP 65 Display : Backlit dot matrix LCD Operating Condition : -20 to +40 deg. C 7020 Analyzer Accuracy : ppm= +/-0. 2 ppm ; ppb=2 ppb or 5%of reading Advance feature : Auto Ranging of Input, Auto Calibration & Cleaning relays & software, Control function Operating Voltage : 16 -24 Vdc power looped Output Signal : 4 -20 m. A

Honeywell DO Instrumentation Model : DL 424 ppm/DL 425 ppb Case : Plastic Display : Backlit dot matrix LCD Operating Condition : -20 to +60 deg. C Accuracy : ppm= +/-0. 2 ppm ; ppb=2 ppb or 5%of reading Advance feature : Integral electronics/sensor Operating Voltage : 16 -24 Vdc power looped Direct. Line® Output Signal : 4 -20 m. A

Example Application 7020 With Automatic Clean and Calibration

Direct. Line® § Easy to install/set-up: - Direct. Line architecture i. e. integral sensor/electronics reduce wiring, cable runs and panel cut-outs § Easy-to-use: - Local display and keypad provides easy set-up, calibration and operation Calibration can be done right at electrode, no running back and forth to analyzer (convenient + time + money savings) § Easy Maintenance: - Plug-in sensors allow electrode/probe replacement in few minutes; no wiring required (convenient + time + money savings)

Direct. Line® Remote Type Electronic Module Pre-Amp Module Remote Cable Shield Twist Pair 20’/100’ DO Electrode

Thank Dissolved You Oxygen (DO)