What is Density is the amount of matter









- Slides: 16

What is Density? • is the amount of matter in a specific volume. • is measured in g/cm 3 or g/m. L • can be used to help identify an

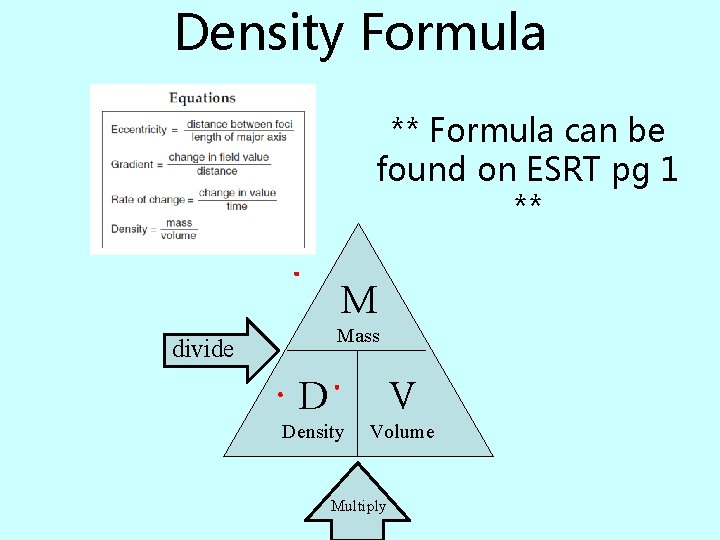
Density Formula ** Formula can be found on ESRT pg 1 ** M Mass divide D V Density Volume Multiply

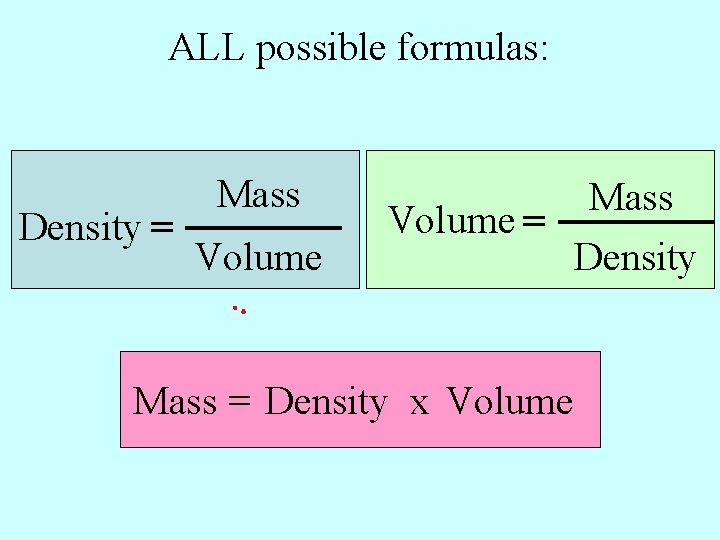
ALL possible formulas: Density = Mass Volume = Density Mass = Density x Volume

** Density of an object remains the same no matter how many pieces it’s broken in to! ** * The density of an object DOES NOT change if you change its mass!* Therefore, we can find an object's density to help identify various materials. Ex. The density of 20. 0 g of aluminum = 2. 7 g/cm 3 The density of 99. 0 g of aluminum = 2. 7 g/cm 3

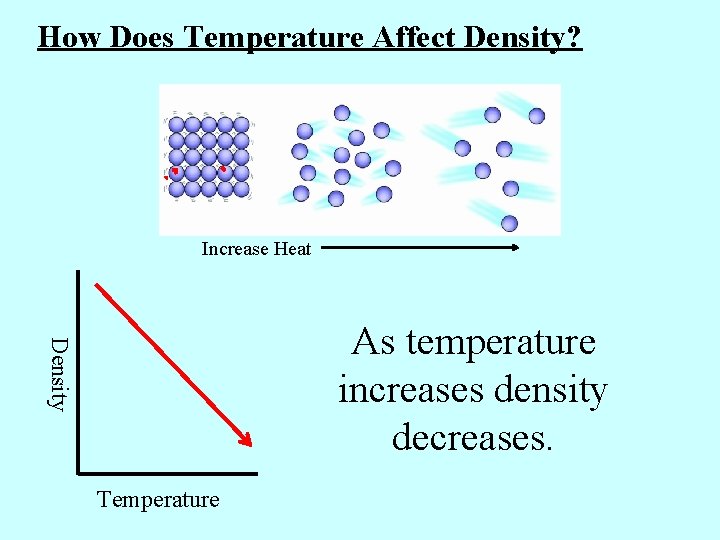
How Does Temperature Affect Density? Increase Heat Density As temperature increases density decreases. Temperature

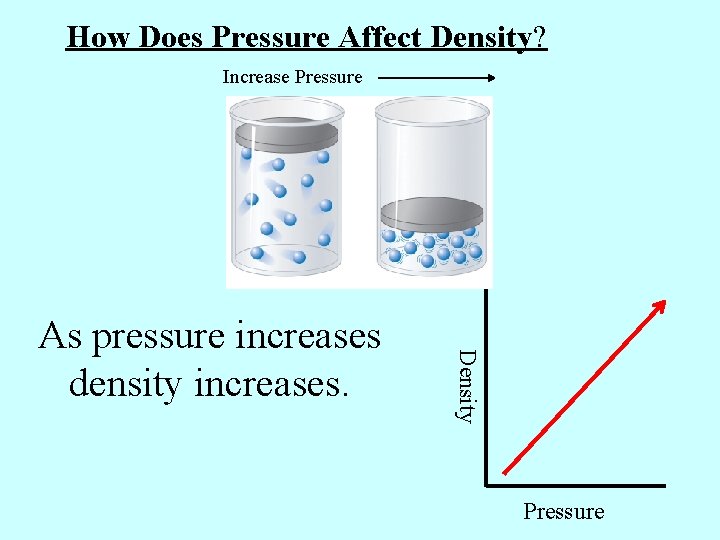
How Does Pressure Affect Density? Increase Pressure Density As pressure increases density increases. Pressure

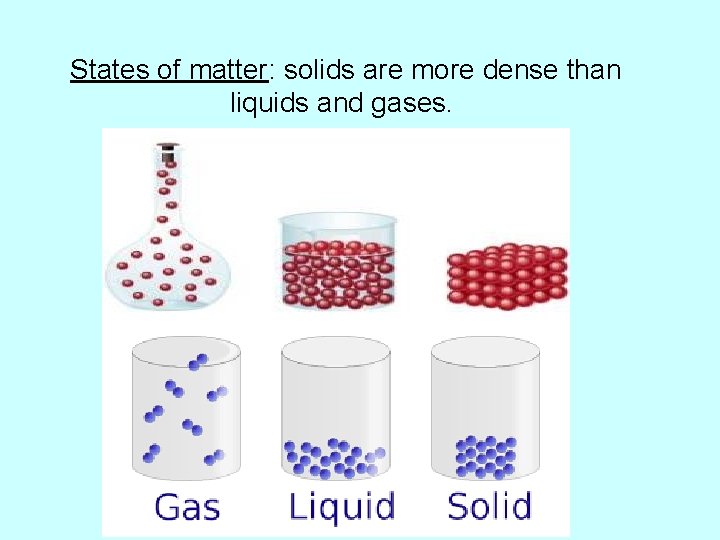
States of matter: solids are more dense than liquids and gases.

*except water – most dense at 4 deg. Celsius (C) as a liquid

Less dense materials float. More dense materials sink.

Practice Problems 1. What is the density of on object with a mass of 120 g and a volume of 7 m. L? 2. What is the volume of 22 O grams of on object with a density of 55 g/cm 3 ?

3. A block of wood has a mass of 180 grams. It is 10. 0 cm long, 6. 0 cm wide, and 4. 0 cm thick. What is its volume and density?

Now Try These. . . Solve the following (don’t forget units): 1. M= 34. 1 g V= 78. 5 m. L Density = ______ 2. M= 27 g D=. 76 g/cm 3 Volume = ______

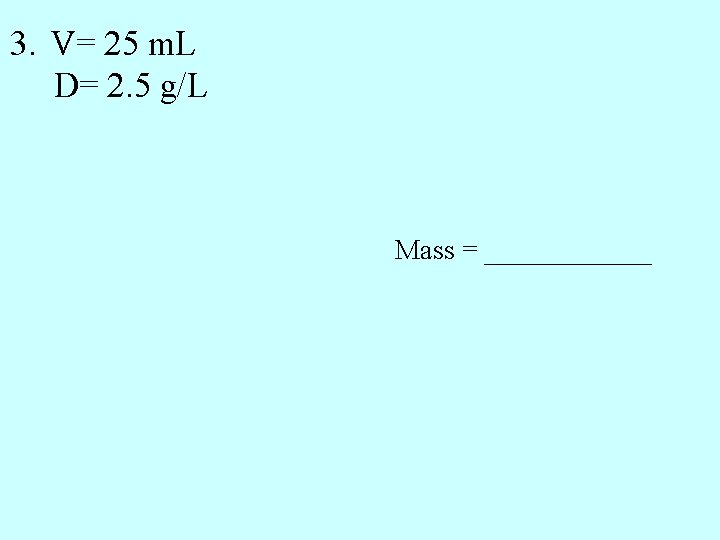
3. V= 25 m. L D= 2. 5 g/L Mass = ______

Example: Compare the density of H 2 O (water) to the density of an ice cube. THINK – If an ice cube floats in H 2 O because it has a density: a) Equal to the density of water b) Greater than the density of water c) Less than the density of water d) None of the above

THINK: Why does a steel ship float in water? Densities in grams / cm 3 D (water) = 1. 0 g / cm 3 D (wood) = 0. 8 g / cm 3 D (steel) = 7. 8 g / cm 3 D (lead) 11. 3 g / cm 3 D (air). 0012 g / cm 3

Answer: A ship has a steel shell that is hollow inside. The volume is mostly air. The ship and air together have a density less than water. Density Worksheet