What is density Density is how heavy something







- Slides: 16


What is density? Density is how heavy something is for its size. It's how much it weighs divided by how big it is. So, something that's very heavy and small has a high density. Density is mass divided by volume. To work out the density of something, divide mass (how much it weighs), by volume (how much space it takes up).

Density is a measure of mass per volume. That is, density measures how much matter is in a given volume. Density = . mass . volume Density is usually measured in g/m. L or kg/L for liquids and gasses and in g/cm 3 for solids.

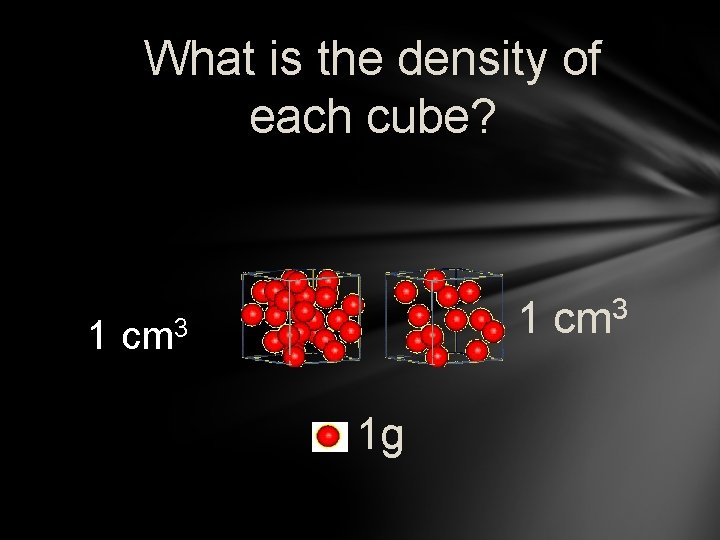
What is the density of each cube? 1 1 cm 3 1 g 3 cm

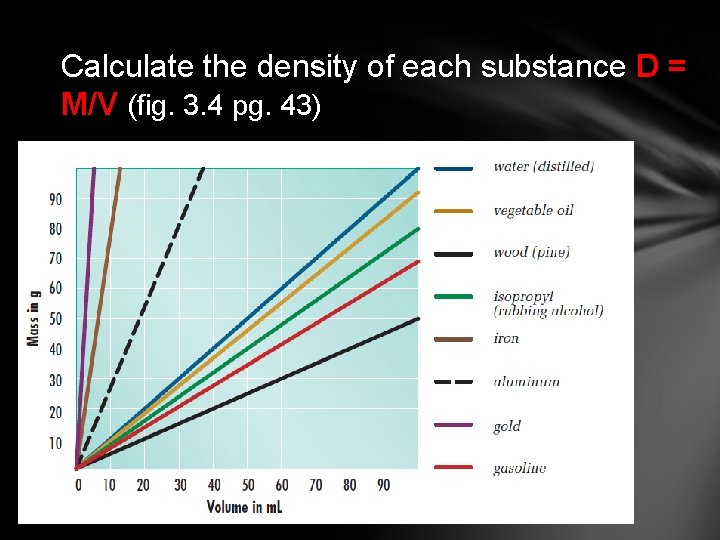
Calculate the density of each substance D = M/V (fig. 3. 4 pg. 43)

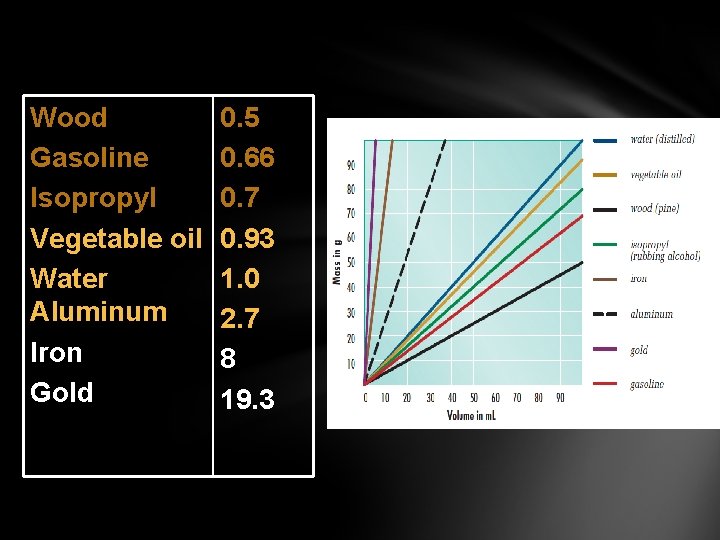
Wood Gasoline Isopropyl Vegetable oil Water Aluminum Iron Gold 0. 5 0. 66 0. 7 0. 93 1. 0 2. 7 8 19. 3

Floating Grape Demo. . . You have 2 cups of the same amount of water. Salt is added to one cup of water. When a grape is added to both cups of water, what do you think is going to happen to the grape in each instance? Will it sink? Float? The cup of water that has salt added to it is MORE dense then the grape that was added to that cup of water. The grape is LESS dense then the saltwater solution.

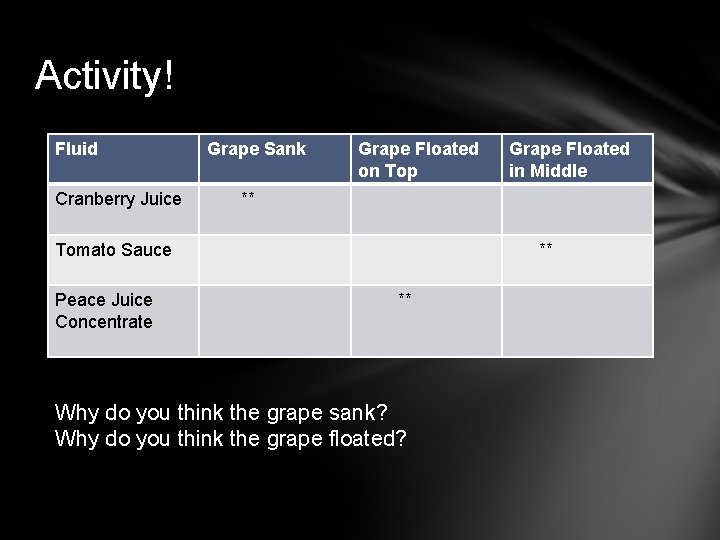
Activity! Fluid Cranberry Juice Grape Sank Grape Floated on Top ** Tomato Sauce Peace Juice Concentrate Grape Floated in Middle ** ** Why do you think the grape sank? Why do you think the grape floated?

What is Density? • Density is a property of fluids. It is the amount of matter in a given volume. • Density can be thought of as a measure of the ‘heaviness’ of an object. • Something that is very heavy and small has a HIGH density! • Example: An apple will feel lighter then a potato of the same size because the apple is less dense then the potato. • Example: A golf ball has more mass per unit of volume then a ping pong ball does.

Understanding Density • Not all substances have the same density. • Recall that the Particle Model of Matter states that all matter is made up of tiny particles and different substances are made of different particles. • So, the density of a substance depends on the particles it is made of. • Density is a measure of how tightly packed or how heavy the particles are in a substance.

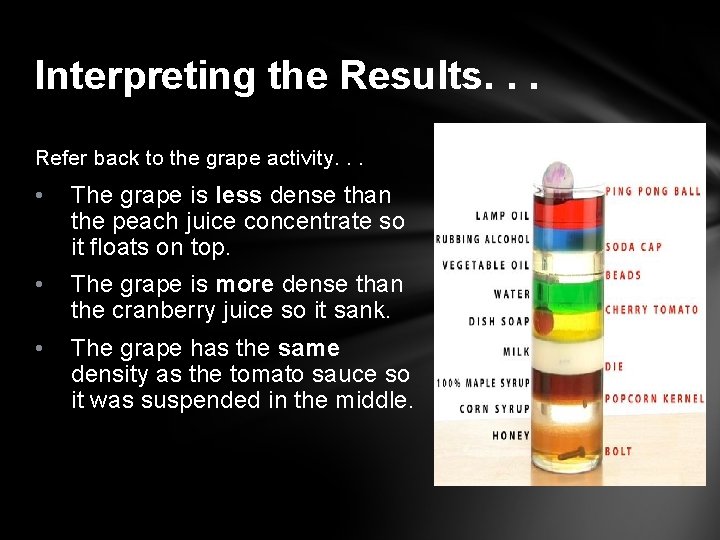
Interpreting the Results. . . Refer back to the grape activity. . . • The grape is less dense than the peach juice concentrate so it floats on top. • The grape is more dense than the cranberry juice so it sank. • The grape has the same density as the tomato sauce so it was suspended in the middle.

States of Matter and Density • Density of a substance is different in different states. • Solid and liquid states of a substance have similar densities. • Gas state may have a range of densities depending on how much the gas is compressed. • Most fluids become denser as they cool down to their freezing temperature. (When particles cool down, they move less, are closer together, and therefore heavier)

The Weird Case of Water • The fluid that is the exception to this rule is water. • Its greatest density is at 4 degrees Celsius (1 g/ml) • Water becomes less dense as the temperature drops from 4 degrees to 0 degrees Celsius. • This is why ice floats.

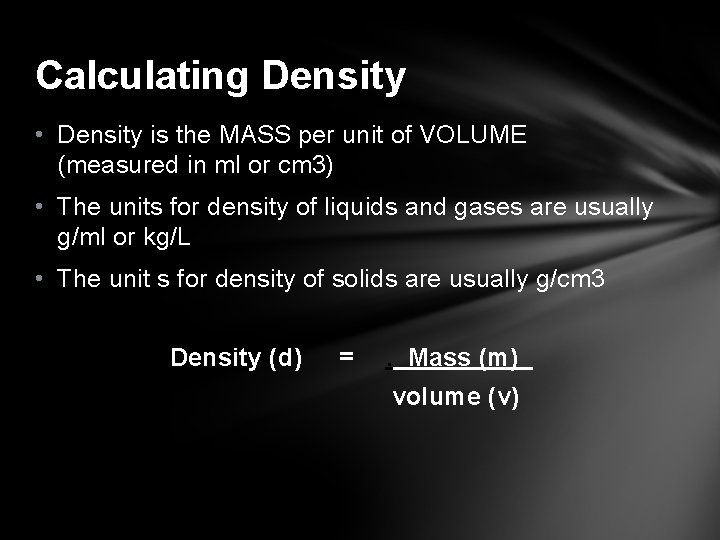
Calculating Density • Density is the MASS per unit of VOLUME (measured in ml or cm 3) • The units for density of liquids and gases are usually g/ml or kg/L • The unit s for density of solids are usually g/cm 3 Density (d) = . Mass (m) volume (v)

Review! Answer these questions with a partner. . . 1. What is density? 2. What is the formula for density? 3. What happens if you pour together liquids that have different densities? 4. Will the liquid on the top have the highest or lowest density? 5. Will the liquid on the bottom have the highest or lowest density?

Check and Reflect page 46 #’s 1 -5