What is CO 2 friend or foe Tom













- Slides: 29

What is CO 2 – friend or foe? Tom V. Segalstad Head of the Geological Museum, Natural History Museum, University of Oslo http: //folk. uio. no/tomvs

Atmosphere gases The Earth's atmosphere contains on wet basis ~73. 5 % nitrogen, ~22. 5 % oxygen, ~2. 7 % water, and ~1. 25 % argon per weight. Among the trace gases are: CO 2, neon, helium, methane, and others. The content of CO 2 is ca. 0. 05 weight-%, compared with ca. 2. 7 weight-% water.

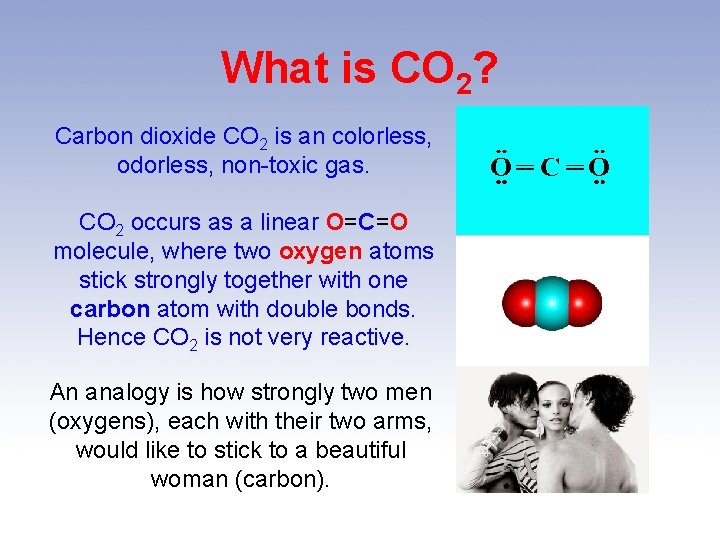
What is CO 2? Carbon dioxide CO 2 is an colorless, odorless, non-toxic gas. CO 2 occurs as a linear O=C=O molecule, where two oxygen atoms stick strongly together with one carbon atom with double bonds. Hence CO 2 is not very reactive. An analogy is how strongly two men (oxygens), each with their two arms, would like to stick to a beautiful woman (carbon).

Some daily life uses of CO 2 • Fire extinguishers (replacement of oxygen) • Baking soda (expansion of non-toxic gas) • Soda ”pop” drinks, beer, champagne (effervescense; added or from fermentation) • Neutralizing agent for acid lakes (limestone) • Life jackets (gas expansion) • Cooling agent • Product of our breathing! CH 2 O + O 2 CO 2 + H 2 O carbohydrate + oxygen CO 2 + water

Plant photosynthesis consume CO 2 Plants make carbohydrate by combining atmospheric CO 2 with water, powered by light: photosynthesis CO 2 + H 2 O + energy CO 2 + water + energy CH 2 O + O 2 carbohydrate + oxygen breathing, decay Increasing CO 2, water and energy will make the chemical reaction go from left to right, making the plants produce more carbohydrates. We need for living carbohydrates made by plants. Hence CO 2 is: THE GAS OF LIFE !

Consequence of photosynthesis The photosynthesis / breathing+decay reaction CO 2 + H 2 O + energy CH 2 O + O 2 shows us that all CO 2 accumulated by the plant, will be released again to the atmosphere when the plant material rot or is burned. Then tree planting will only temporarily remove CO 2 from the atmosphere, unless the trees are somehow buried to prevent them from decay or being burned.

IPCC TAR 2001: CO 2 in air (upper graph) and surface temperatures (lower graph) were constant for some 900 years, but have risen considerably the last 100 years. IPCC’s scenarios involve drastic rises in both air CO 2 concentration and surface temperatures. These assertions have been strongly opposed by CO 2 measurement critics and historical temperature facts: warm + cold missing. Medieval warm period missing called the ”Climate Optimum” The ”Little Ice Age” missing

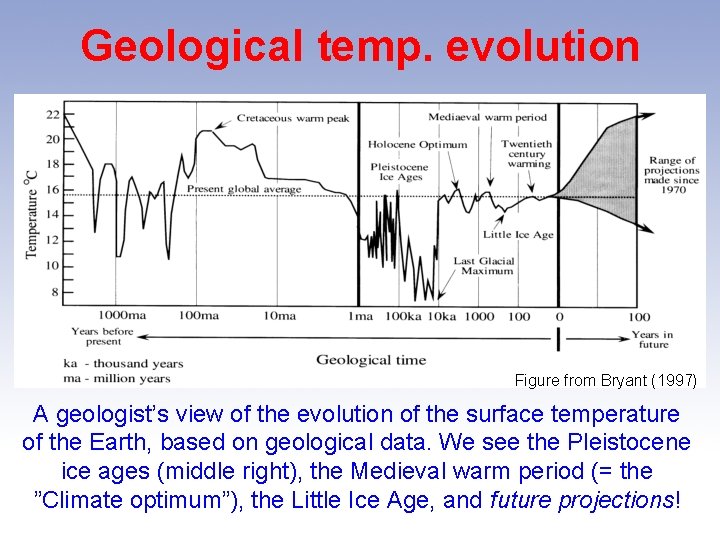
Geological temp. evolution Figure from Bryant (1997) A geologist’s view of the evolution of the surface temperature of the Earth, based on geological data. We see the Pleistocene ice ages (middle right), the Medieval warm period (= the ”Climate optimum”), the Little Ice Age, and future projections!

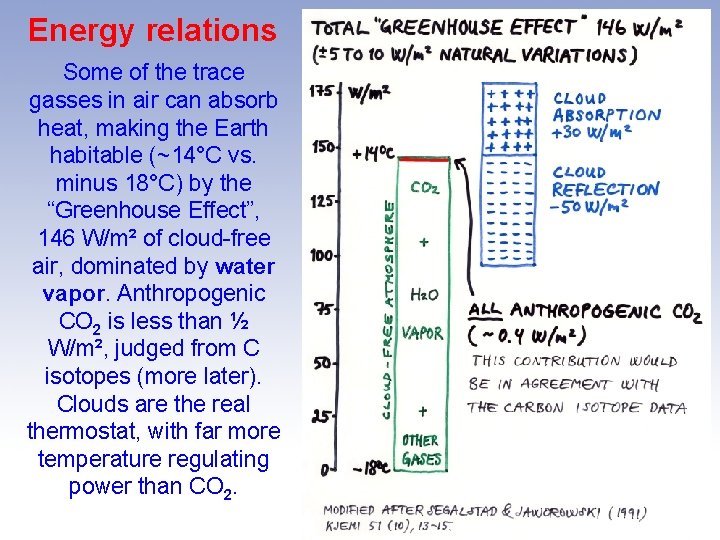
Energy relations Some of the trace gasses in air can absorb heat, making the Earth habitable (~14°C vs. minus 18°C) by the “Greenhouse Effect”, 146 W/m² of cloud-free air, dominated by water vapor. Anthropogenic CO 2 is less than ½ W/m², judged from C isotopes (more later). Clouds are the real thermostat, with far more temperature regulating power than CO 2.

Other energy relations All ice on Earth has a mass of 3. 3 x 1022 g. Its latent heat of fusion is 9. 3 x 1024 J. The Earth’s ocean has a mass of 1. 4 x 1024 g. Assertions say that ”all ice on Earth will melt in a short time from anthropogenic CO 2”. If melting energy hypothetically had been taken from the ocean, all its water would cool 2°C. Heat-absorbing part of the air has a mass of only 1. 4 x 1022 g. Heating all of the atmosphere 2°C would require energy of 1. 2 x 1022 J. This amount of energy is not enough to first heat the air over the poles to the melting point of ice (0°C) and next to overcome the latent heat of fusion for all ice on Earth. Thus ice and ocean participace as “thermostats”.

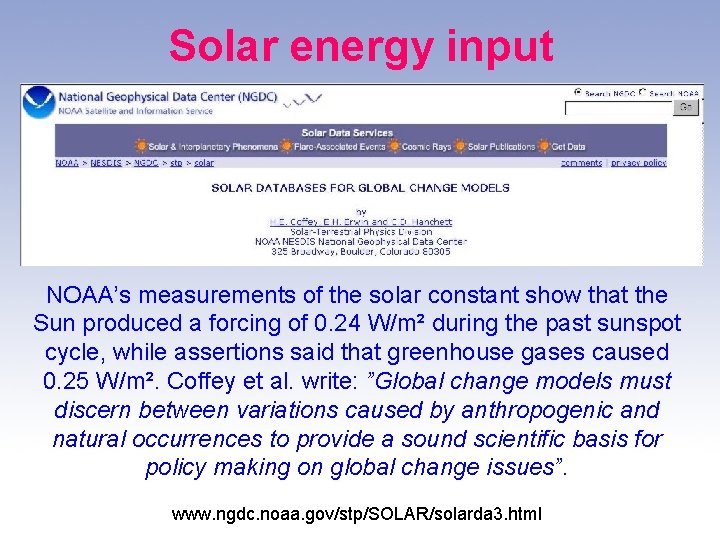
Solar energy input NOAA’s measurements of the solar constant show that the Sun produced a forcing of 0. 24 W/m² during the past sunspot cycle, while assertions said that greenhouse gases caused 0. 25 W/m². Coffey et al. write: ”Global change models must discern between variations caused by anthropogenic and natural occurrences to provide a sound scientific basis for policy making on global change issues”. www. ngdc. noaa. gov/stp/SOLAR/solarda 3. html

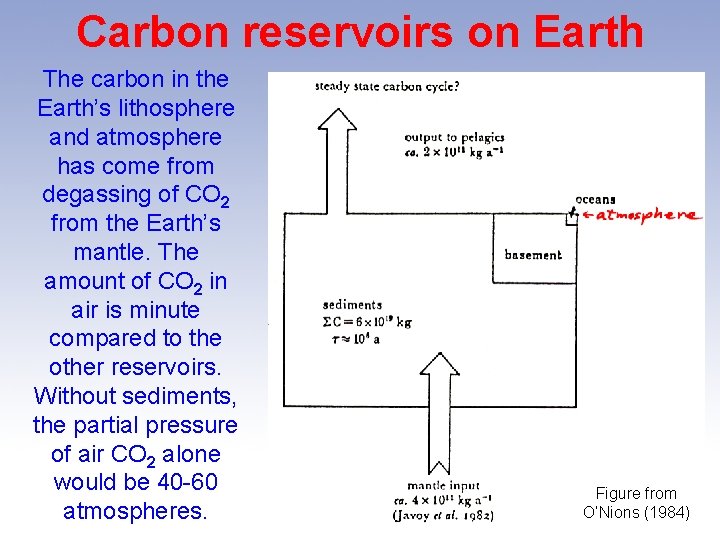
Carbon reservoirs on Earth The carbon in the Earth’s lithosphere and atmosphere has come from degassing of CO 2 from the Earth’s mantle. The amount of CO 2 in air is minute compared to the other reservoirs. Without sediments, the partial pressure of air CO 2 alone would be 40 -60 atmospheres. Figure from O’Nions (1984)

INORGANIC CARBON CYCLE This review is important; IPCC’s ocean is clean distilled water. CO 2 enters the atmosphere from many sources to the left. Atmospheric CO 2 dissolves, hydrolyses and protolyses in the ocean. CO 2 may combine with calcium and precipitate as Ca. CO 3 in limestone, sedimented on the sea floor together with shells from organisms. This is analogous to breathing CO 2 into a test tube with Ca(OH)2; Ca. CO 3 almost instantly precipitates.

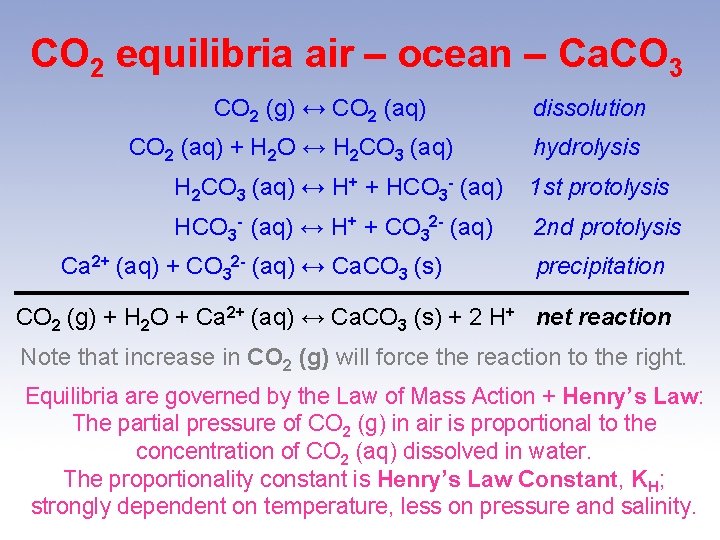
CO 2 equilibria air – ocean – Ca. CO 3 CO 2 (g) ↔ CO 2 (aq) + H 2 O ↔ H 2 CO 3 (aq) dissolution hydrolysis H 2 CO 3 (aq) ↔ H+ + HCO 3 - (aq) 1 st protolysis HCO 3 - (aq) ↔ H+ + CO 32 - (aq) 2 nd protolysis Ca 2+ (aq) + CO 32 - (aq) ↔ Ca. CO 3 (s) precipitation CO 2 (g) + H 2 O + Ca 2+ (aq) ↔ Ca. CO 3 (s) + 2 H+ net reaction Note that increase in CO 2 (g) will force the reaction to the right. Equilibria are governed by the Law of Mass Action + Henry’s Law: The partial pressure of CO 2 (g) in air is proportional to the concentration of CO 2 (aq) dissolved in water. The proportionality constant is Henry’s Law Constant, KH; strongly dependent on temperature, less on pressure and salinity.

Henry’s Law in daily use Henry’s Law Constant is an equilibrium partition coefficient for CO 2 (g) in air vs. CO 2 (aq) in water: at 25°C KH ≈ 1 : 50 At lower temperature more gas dissolves in the water. We have all experienced this – cold soda or beer or champagne can contain more CO 2; thus has more effervescense than hot drinks. The brewery sais that they add 3 liters of CO 2 to 1 liter of water in the soda. But where did all the CO 2 go?

Henry’s Law in daily use ”atmosphere” ”ocean” Henry’s Law Constant directs that CO 2 (g) in air vs. CO 2 (aq) in water at 25°C is distributed ≈ 1 : 50 This means that there will be about 50 times more CO 2 dissolved in water than contained in the free air above. The soda bottle is a good analogue to nature: there is about 50 times more CO 2 in the ocean than in the Earth’s atmosphere. Ocean water has 120 mg HCO 3 - per liter; as much CO 2 as in 180 liter of air.

The speed of Henry’s Law ”atmosphere” ”ocean” IPCC claims that the CO 2 equilibration between air and water will take 50 - 200 years as ”a rough indication” (IPCC 1990; Table 1. 1). Furthermore that most of the CO 2 added to air will accumulate in the air, and very little be dissolved in water: Table from Segalstad (1998); after Rohde (1992). Experiments show this not to be the case. Do we all wait for 50 – 200 years for our soda or beer from the brewery?

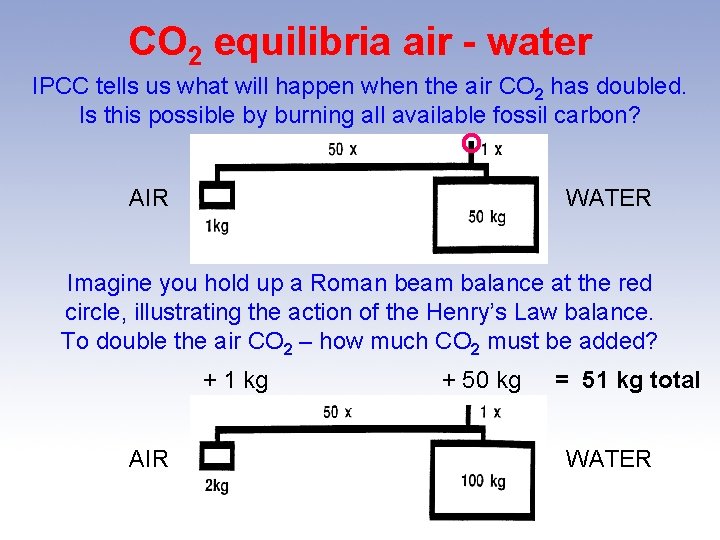
CO 2 equilibria air - water IPCC tells us what will happen when the air CO 2 has doubled. Is this possible by burning all available fossil carbon? O AIR WATER Imagine you hold up a Roman beam balance at the red circle, illustrating the action of the Henry’s Law balance. To double the air CO 2 – how much CO 2 must be added? + 1 kg AIR + 50 kg = 51 kg total WATER

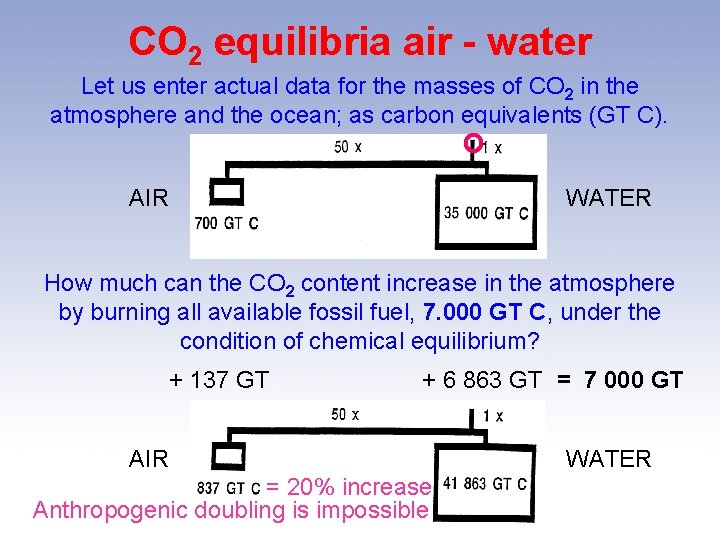
CO 2 equilibria air - water Let us enter actual data for the masses of CO 2 in the atmosphere and the ocean; as carbon equivalents (GT C). O AIR WATER How much can the CO 2 content increase in the atmosphere by burning all available fossil fuel, 7. 000 GT C, under the condition of chemical equilibrium? + 137 GT + 6 863 GT = 7 000 GT AIR = 20% increase Anthropogenic doubling is impossible WATER

IPCC’s proof of anthropogenic global warming • Atmospheric CO 2 increase ”closely parallels” accumulated emissions from the burning of fossil fuels. • CO 2 in ice cores show that air CO 2 has increased 21% from 280 til 353 ppm over the last 150 years. • Carbon isotope ratios of 13 C/12 C and 14 C/12 C have decreased in atmospheric CO 2, they ”agree qualitatively” with expected additions of 12 C from burning of fossil fuel (enriched in 12 C). This implicate that CO 2 has a long lifetime in the Earth’s atmosphere (”rough indication 50 – 200 years”).

IPCC’s proofs rejected In a number of publications our research group has rejected IPCC’s 3 proofs of anthropogenic warming.

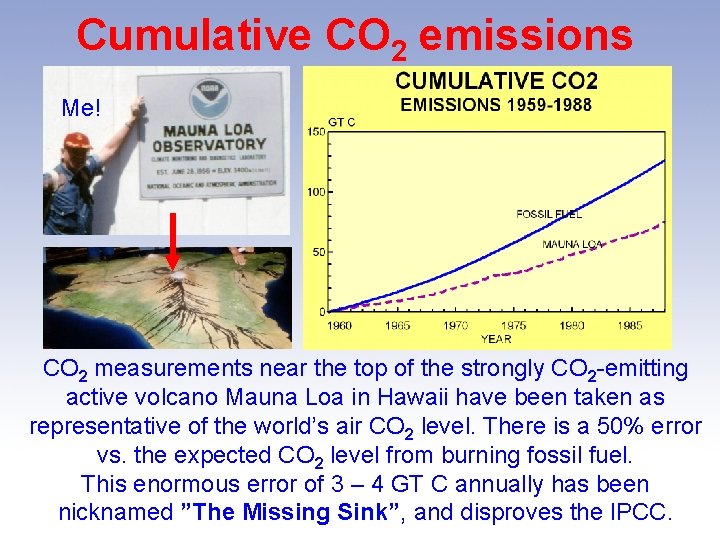
Cumulative CO 2 emissions Me! CO 2 measurements near the top of the strongly CO 2 -emitting active volcano Mauna Loa in Hawaii have been taken as representative of the world’s air CO 2 level. There is a 50% error vs. the expected CO 2 level from burning fossil fuel. This enormous error of 3 – 4 GT C annually has been nicknamed ”The Missing Sink”, and disproves the IPCC.

Stable carbon isotopes 13 C/12 C isotope ratios are expressed as δ (delta) values defined as the standard-normalized difference from the standard, expressed as δ 13 C in per mil (‰). The reference standard used is PDB (Pee Dee Belemnite).

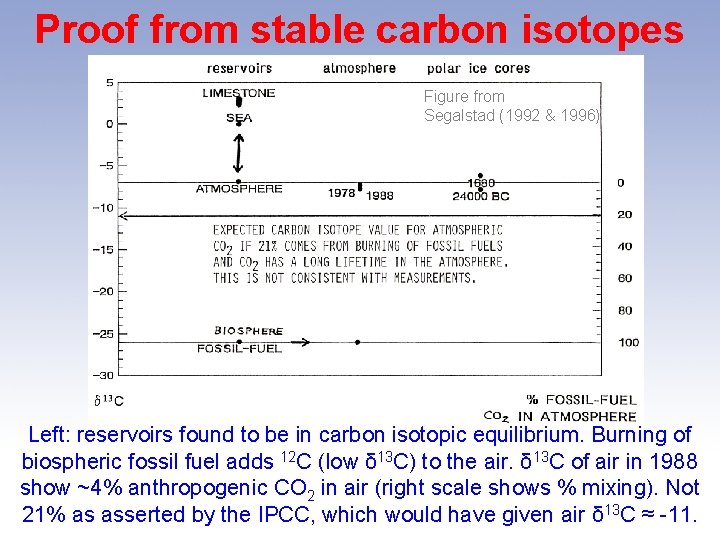
Proof from stable carbon isotopes Figure from Segalstad (1992 & 1996) Left: reservoirs found to be in carbon isotopic equilibrium. Burning of biospheric fossil fuel adds 12 C (low δ 13 C) to the air. δ 13 C of air in 1988 show ~4% anthropogenic CO 2 in air (right scale shows % mixing). Not 21% as asserted by the IPCC, which would have given air δ 13 C ≈ -11.

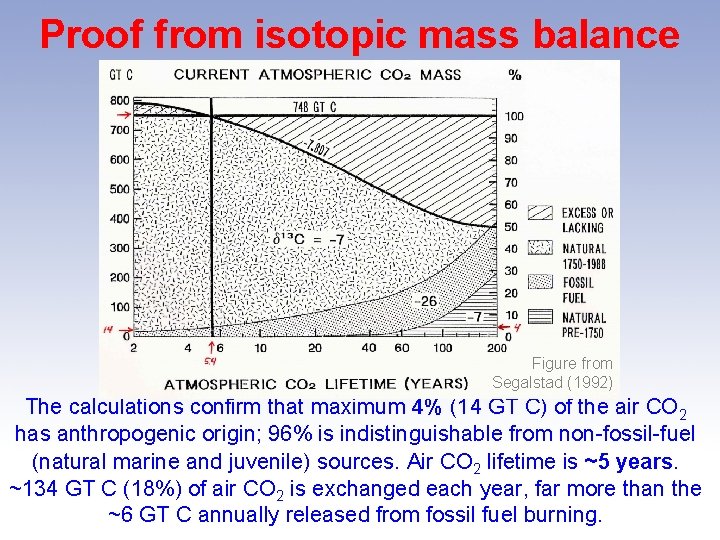
Proof from isotopic mass balance Figure from Segalstad (1992) Using the radioactive decay equation for the lifetime of CO 2 in air, we can calculate the masses of remaining CO 2 from different reservoirs using isotopic mass balance; checking for match vs. air CO 2 in December 1988: mass = 748 GT C; δ 13 C = -7. 807 (Keeling et al. 1989).

Proof from isotopic mass balance Figure from Segalstad (1992) The calculations confirm that maximum 4% (14 GT C) of the air CO 2 has anthropogenic origin; 96% is indistinguishable from non-fossil-fuel (natural marine and juvenile) sources. Air CO 2 lifetime is ~5 years. ~134 GT C (18%) of air CO 2 is exchanged each year, far more than the ~6 GT C annually released from fossil fuel burning.

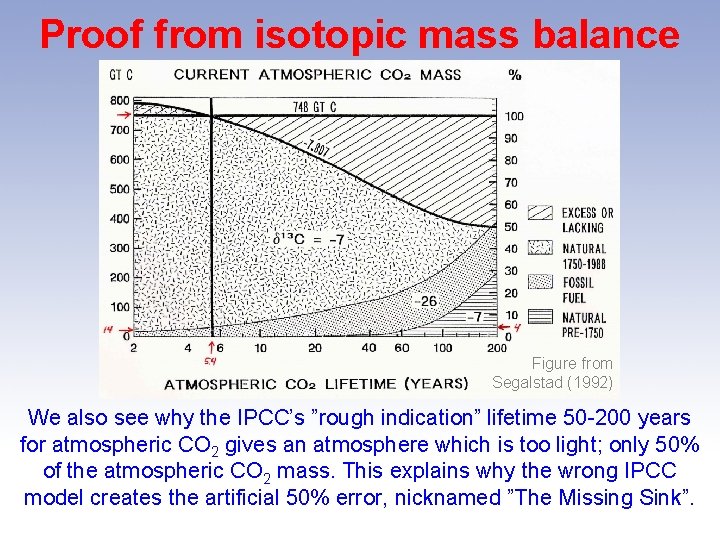
Proof from isotopic mass balance Figure from Segalstad (1992) We also see why the IPCC’s ”rough indication” lifetime 50 -200 years for atmospheric CO 2 gives an atmosphere which is too light; only 50% of the atmospheric CO 2 mass. This explains why the wrong IPCC model creates the artificial 50% error, nicknamed ”The Missing Sink”.

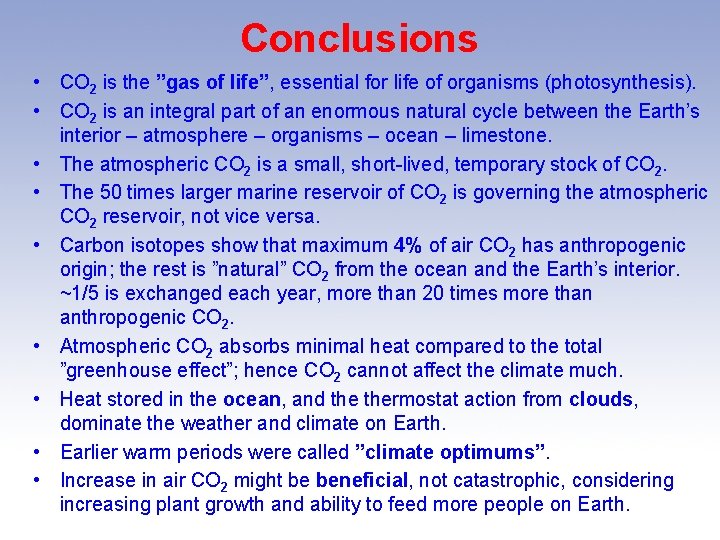
Conclusions • CO 2 is the ”gas of life”, essential for life of organisms (photosynthesis). • CO 2 is an integral part of an enormous natural cycle between the Earth’s interior – atmosphere – organisms – ocean – limestone. • The atmospheric CO 2 is a small, short-lived, temporary stock of CO 2. • The 50 times larger marine reservoir of CO 2 is governing the atmospheric CO 2 reservoir, not vice versa. • Carbon isotopes show that maximum 4% of air CO 2 has anthropogenic origin; the rest is ”natural” CO 2 from the ocean and the Earth’s interior. ~1/5 is exchanged each year, more than 20 times more than anthropogenic CO 2. • Atmospheric CO 2 absorbs minimal heat compared to the total ”greenhouse effect”; hence CO 2 cannot affect the climate much. • Heat stored in the ocean, and thermostat action from clouds, dominate the weather and climate on Earth. • Earlier warm periods were called ”climate optimums”. • Increase in air CO 2 might be beneficial, not catastrophic, considering increasing plant growth and ability to feed more people on Earth.

… spectacular facts are hard to beat …
 Hello my friend hello song
Hello my friend hello song Hello my friend hello my future
Hello my friend hello my future Friend or foe game show
Friend or foe game show Friend or foe chapter 2
Friend or foe chapter 2 Friend or foe station lab answers
Friend or foe station lab answers Foaf friend of a friend
Foaf friend of a friend A friend in needs a friend indeed
A friend in needs a friend indeed I've found a friend oh such a friend
I've found a friend oh such a friend Let's be good friends
Let's be good friends Tomtom go 910 update
Tomtom go 910 update What does tom symbolize in the devil and tom walker
What does tom symbolize in the devil and tom walker Golden crops foe
Golden crops foe Foe joe
Foe joe Calcolatore foe
Calcolatore foe Mmu foe fyp
Mmu foe fyp Foe coetzee summary
Foe coetzee summary Birth of shaka zulu poem
Birth of shaka zulu poem Fee fie foe firm
Fee fie foe firm Pothos statua
Pothos statua Psyici
Psyici Foe mmu
Foe mmu As cannons overcharged with double cracks
As cannons overcharged with double cracks My best friend essay
My best friend essay While maria was cleaning the apartment her husband
While maria was cleaning the apartment her husband 耶穌是我親愛朋友
耶穌是我親愛朋友 Sitcom
Sitcom I can be a super friend
I can be a super friend You said i am feeling ill
You said i am feeling ill Bosom friend anne of green gables
Bosom friend anne of green gables Adt friend finder
Adt friend finder