What is Chemistry THE STUDY OF MATTER AND












- Slides: 21

What is Chemistry? THE STUDY OF MATTER AND THE CHANGES THAT TAKE PLACE WITH THAT MATTER

The Chemistry World

What is Matter? MATTER: ANYTHING THAT HAS MASS AND TAKES UP SPACE Mass – the amount of matter in something Volume – the amount of space something occupies

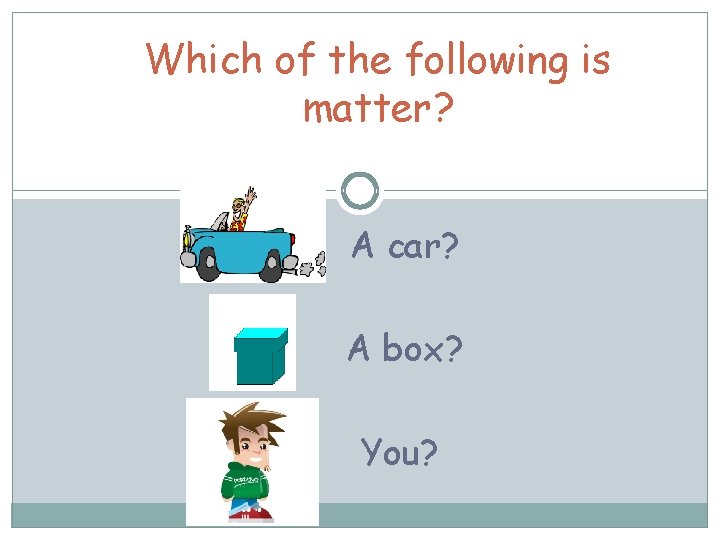
Which of the following is matter? A car? A box? You?

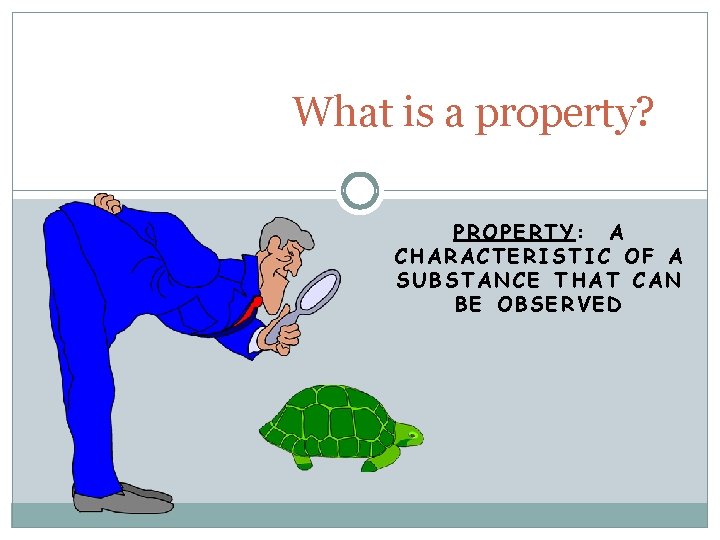
What is a property? PROPERTY: A CHARACTERISTIC OF A SUBSTANCE THAT CAN BE OBSERVED

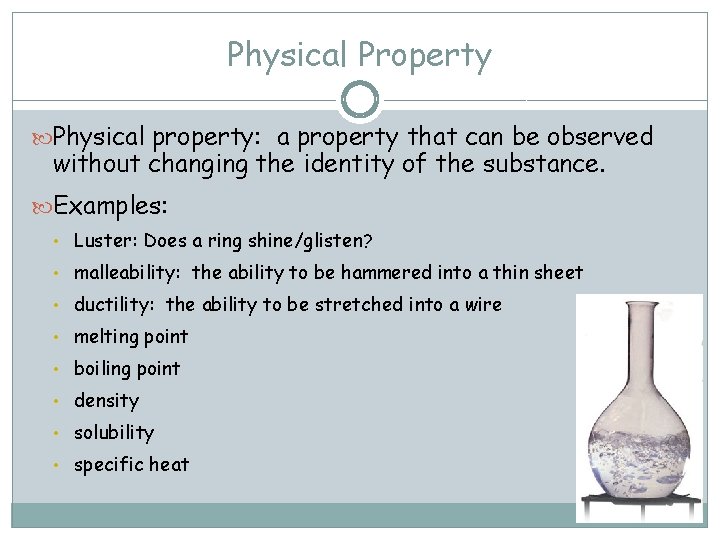
Physical Property Physical property: a property that can be observed without changing the identity of the substance. Examples: • Luster: Does a ring shine/glisten? • malleability: the ability to be hammered into a thin sheet • ductility: the ability to be stretched into a wire • melting point • boiling point • density • solubility • specific heat

Special Physical Properties MELTING POINT: THE TEMPERATURE AT WHICH A SUBSTANCE CHANGES FROM A SOLID TO A LIQUID AT A GIVEN PRESSURE WATER = 0 o. C BOILING POINT: THE TEMPERATURE AT WHICH A SUBSTANCE CHANGES FROM A LIQUID TO A GAS AT A GIVEN PRESSURE WATER = 100 O C

Chemical Property: Characteristics of a material that CANNOT be observed without causing a chemical change Examples: Reacting with Oxygen (oxidation): Iron Rust Apple Turning Brown Reacting with Acids: The formation of compounds (What’s a compounds? ! We will get there!!!) Flammable/Nonflammable

What is a Pure Substance? A sample of matter that has definite chemical and physical properties and cannot be changed into a simpler substance. (it’s homogeneous) Examples: Tin Sulfur Diamond Water Pure Sugar Table Salt

What is a mixture? Combination of two or more pure substances that are NOT chemically combined Substances are held together by physical forces, not chemical No chemical change takes place Each item keeps its properties in the mixture Can be separated physically

2 Types of Mixtures Homogeneous Mixtures Molecules are mixed up in an even distribution Solutions are mixtures that appear to be in a single substance Heterogeneous Mixtures Molecules are NOT mixed up in an even distribution Parts can be filtered out of the mixture

Examples of Homogeneous Mixtures Sugar water Lemonade Kool-Aid Soda Air

Examples of Heterogeneous Mixtures Snow globe Sand in a bucket of water Muddy water Italian Salad Dressing

What is an Element? Pure substances that cannot be separated into simpler substances Elements are composed of ONE kind of atom Elements are the simplest substances All the elements on earth can be found on the Periodic Table Examples: Oxygen (O), Carbon (C), Hydrogen (H), Nitrogen (N)

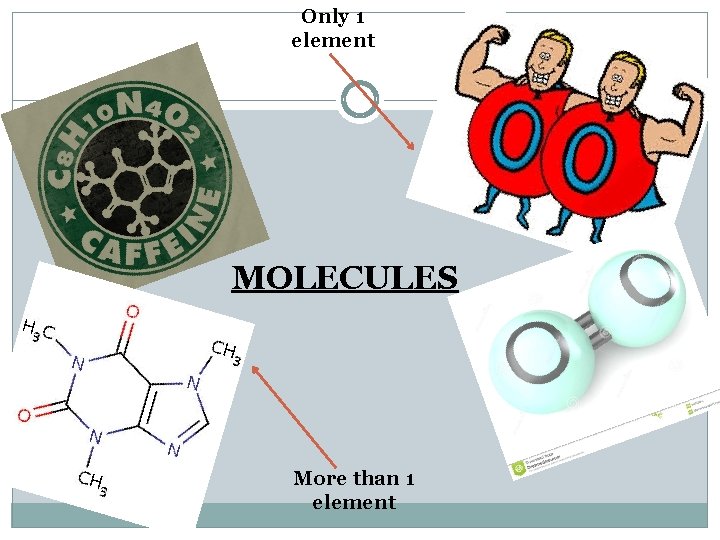
What is a Molecule? Composed of two or more elements that are joined by a chemical bond. Elements can be the same: Ex: H 2, O 2, N 2 Elements can be different: Ex: C 6 H 12 O 6, H 2 O

Only 1 element MOLECULES More than 1 element

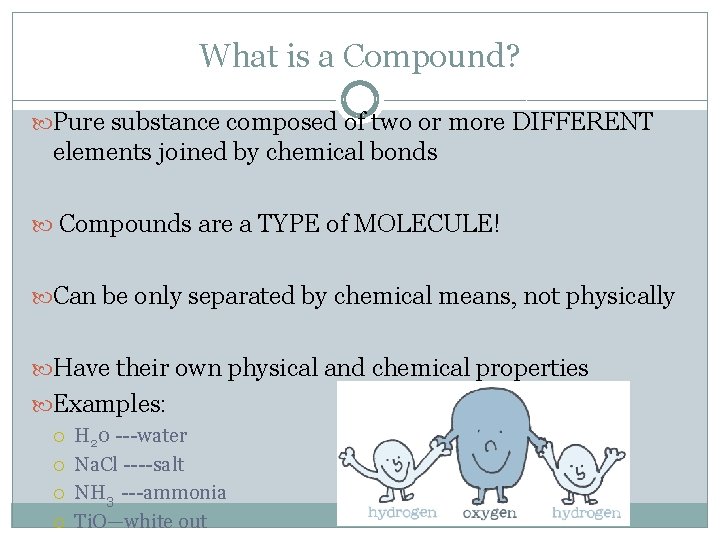
What is a Compound? Pure substance composed of two or more DIFFERENT elements joined by chemical bonds Compounds are a TYPE of MOLECULE! Can be only separated by chemical means, not physically Have their own physical and chemical properties Examples: H 20 ---water Na. Cl ----salt NH 3 ---ammonia Ti. O—white out

ANALOGY Imagine going to an ice cream store. Let's say that they have 30 different flavors of ice cream. Those are elements, the things that I have available to build my dessert from. The smallest amount of ice cream that the store will sell to me is a scoop. This is an atom. If I want, I can put two or more scoops of ice cream together. This is a molecule. If my molecule has more than one flavor of ice cream, I can call it a compound.

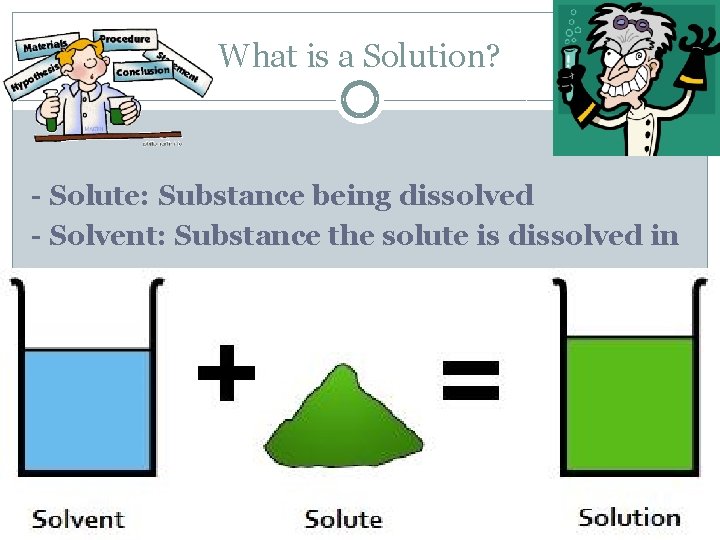
What is a Solution? - Solute: Substance being dissolved - Solvent: Substance the solute is dissolved in

STC LESSON 1: The Nature of Matter OBJECTIVES: 1. DISCUSS YOUR UNDERSTANDING OF THE TERMS PURE SUBSTANCE AND MIXTURE. 2. OBSERVE MATTER IN THE FORM OF PURE SUBSTANCES AND MIXTURES 3. USE YOUR OWN WORDS AND IDEAS TO EXPLAIN YOUR OBSERVATIONS NOTE: **MAKE SURE YOU READ ALL INSTRUCTIONS AND FOLLOW ALL PROCEDURES EXACTLY THROUGHOUT THE LESSON** WE WILL WALK THROUGH LESSON 1. 1 TOGETHER THIS TIME!!!

STC LESSON 2: Pure Substance or Mixture? OBJECTIVES: 1. DISCUSS YOUR UNDERSTANDING OF THE TERMS “PURE SUBSTANCE”. 2. DISCUSS HOW YOU DISTINGUISH BETWEEN PURE SUBSTANCES AND MIXTURES. 3. USE YOUR OWN TECHNIQUES TO DISCOVER WHETHER SEVERAL SAMPLES OF MATTER ARE PURE SUBSTANCES OR MIXTURES NOTE: **MAKE SURE YOU READ ALL INSTRUCTIONS AND FOLLOW ALL PROCEDURES EXACTLY THROUGHOUT THE LESSON**