What is Chemistry Chemistry the science that investigates











- Slides: 13


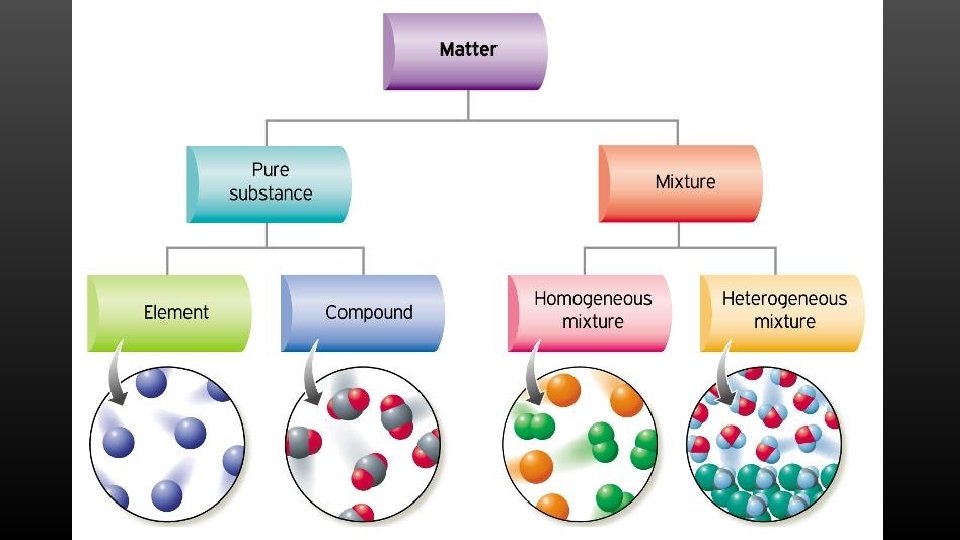
What is Chemistry? • Chemistry – the science that investigates and explains the structure and properties of matter. • Matter – anything that takes up space and has mass • Mass – the measure of the amount of matter in an object

Properties of matter describe the characteristics and behavior of matter, including the changes that it undergoes. Scientists like to classify matter: Qualitative – observation made without measurement Quantitative – observation made with measurement

What is the difference? What are some examples of each?

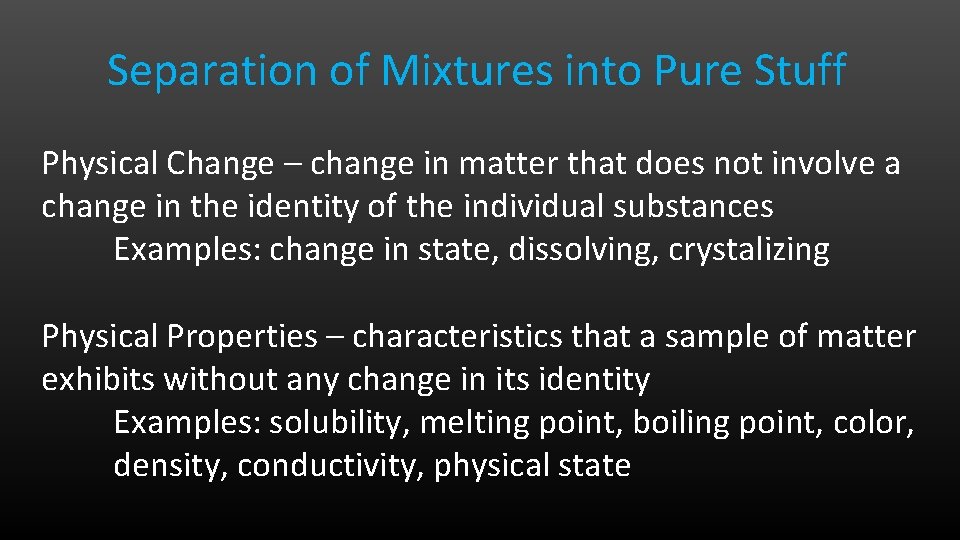
Separation of Mixtures into Pure Stuff Physical Change – change in matter that does not involve a change in the identity of the individual substances Examples: change in state, dissolving, crystalizing Physical Properties – characteristics that a sample of matter exhibits without any change in its identity Examples: solubility, melting point, boiling point, color, density, conductivity, physical state


Homogeneous Mixtures Solution: mixture that is the same throughout Solute - substance being dissolved Solvent – substance that dissolves the solute What are some examples of solutions? Alloys: solid solutions that contain different metals and sometimes nonmetallic substances

Substances: Pure Matter Element – substance that can not be broken down into simpler substances. Elements are the simplest form of matter

Compounds A compound is a chemical combination of two or more different elements joined together in a fixed proportion Formula – chemical symbols and subscripts that show the chemicals in the compound and the ratio in which they are combined

Properties and Changes of Matter - Every substance has a unique set of physical and chemical properties STATES OF MATTER

Chemical Properties and Change Chemical Properties – properties that can be observed only when there is a change in the composition of the substance Examples: rusting, flammability, lack of reactivity, instability Chemical Change (Chemical Reaction) – the of one or more substances into other substances

Chemical Reactions and Energy • Energy – the capacity to do work. Work is done whenever something is moved • Endothermic Reaction – reaction that absorbs or requires energy • Exothermic Reaction – reaction that gives off energy. This can be in the form of heat, light, motion, etc.

Penny Lab • Get a book and turn to page 38 • Put everything except book, lab notebook and writing utensil under desk • Gather your supplies • Start the lab, record all steps and data in your lab notebook
 Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block xoang nhĩ là gì
Block xoang nhĩ là gì Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Talk about your favorite subject
Talk about your favorite subject Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể

























