What is Chemistry Chemistry is the study of





















- Slides: 33

What is Chemistry ?

§ Chemistry is the study of all substances and the changes that they can undergo. § Chemistry has been called the central science because it overlaps many other sciences. § Biologists, Geologists, etc. all use chemistry in their work.

What is Chemistry ? § Chemistry is wherever there is change.

Chemistry § Hair stylists and construction workers need knowledge of some chemistry.

Chemistry cont’d § Hairstylists use the same chemicals found in many chemical labs to get their desired results. § Chemistry is used to 1) Examine a wetlands habitat. 2) Preserve historical artifacts.

Scientific Method § What is the scientific method ? The scientific method is a way of answering questions about the world we live in. § § Make an observation Come up with a question & produce a hypothesis Test it with an experiment Analyze your experiments data to see if it agreed with your hypothesis or theory. § Restructure your experiment and try again. § Continue with the above steps until it becomes a Law.

Hypothesis vs. Theory § What is the difference between a hypothesis and a theory ? § A hypothesis is a first thought about the solution to the problem. It usually doesn’t have any experimental proof. § A theory is developed after some experimentation is done and so it has some evidence to back it up. § A theory comes after the hypothesis.

A Scientific Law § So when does a theory become a Law ? § Let’s take a look at Newton’s Law of Gravity. § He quickly proved that there was a definite relationship in the rate of falling objects. § It became a Law when he was able to describe that relationship so well, that other people were able to duplicate what he did and get the same results.

More Scientific Method § In an experiment, there is a variable: which is the factor being tested. § Experimental control: control responds in a predictable way to the experiment. § Only the variable is allowed to change in a good experiment.

Here’s an example ! § Suppose I wanted test scores to go up. So I made the next test all long essay problems. When I graded the next test, the grades did go up. § CAN I BE CERTAIN THAT IT WAS BECAUSE OF THE ESSAY TESTS ?

The Answer ? ? § The answer is no. § Other variables were also changing. § The test was over other material so this chapter just could have been easier than the last one. § The students could have studied more. § More time could have been spent on this chapter. § All of the other variables would have had to remain the same to be certain as to the reason for the improved scores.

Experiments § Experiments include thought and variables.

Lab Safety § Safety in the lab is of utmost importance: § Failure to follow lab safety rules will result in a failing score for the lab and perhaps further penalties. § There are many lab rules. Here are some of the more important ones. -Follow directions at all times. -Notify if any problems -Wear safety goggles at all times -If it’s hot, let it cool -Carry chemicals with caution -Dispose of chemical wastes properly


Measuring in Science § Metric System: International system of measurement. -Length: Meter (m) -Mass and Weight: Kilogram (kg) -Area and Volume: derived units. Cubic meter (m 3) ex- area rectangle: length X width

More Measurements

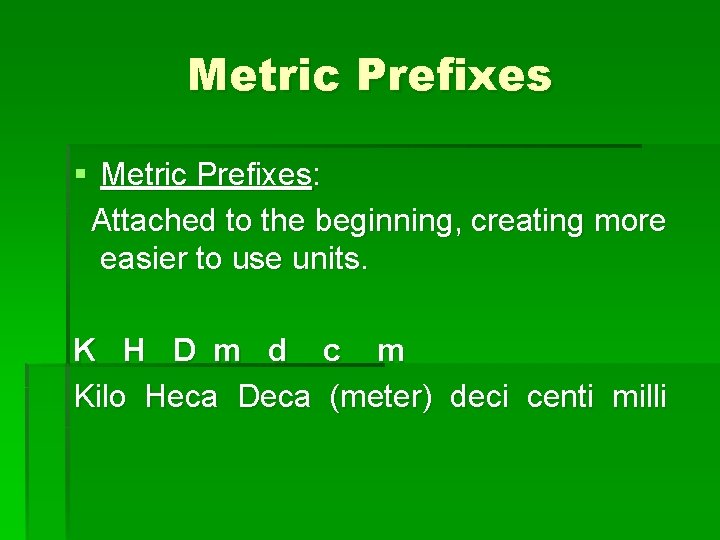
Metric Prefixes § Metric Prefixes: Attached to the beginning, creating more easier to use units. K H D m d c m Kilo Heca Deca (meter) deci centi milli

Uncertainty § Uncertainty in measurement: Measuring is never completely free of flaws and always involves some estimations.

Precision v. Accuracy § Precision: -A reliable measurement will give about the same result again and again under the same conditions. - Accuracy: A result that is close to the accepted value. Ex: A dart board.

Precision and Accuracy § This is an example of precision and accuracy, where the darts land on the board.

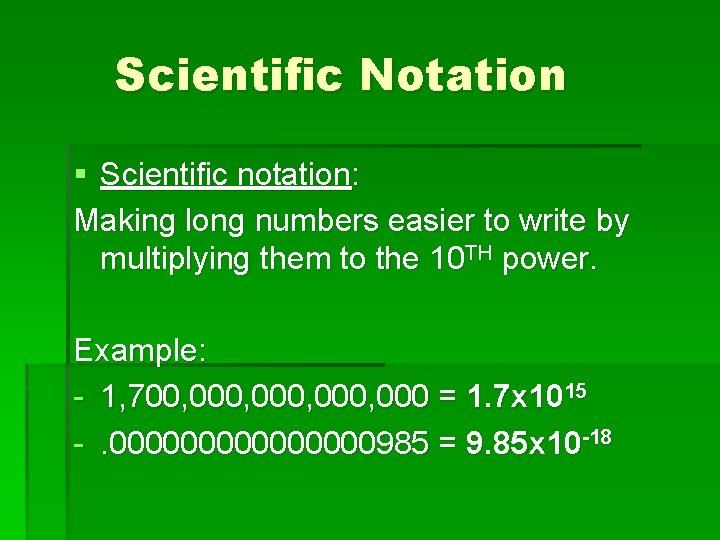
Scientific Notation § Scientific notation: Making long numbers easier to write by multiplying them to the 10 TH power. Example: - 1, 700, 000, 000 = 1. 7 x 1015 -. 00000000985 = 9. 85 x 10 -18

Percent Error § Percent error: Percent error= Measured value * accepted value X 100% -ratios: speed= distance time

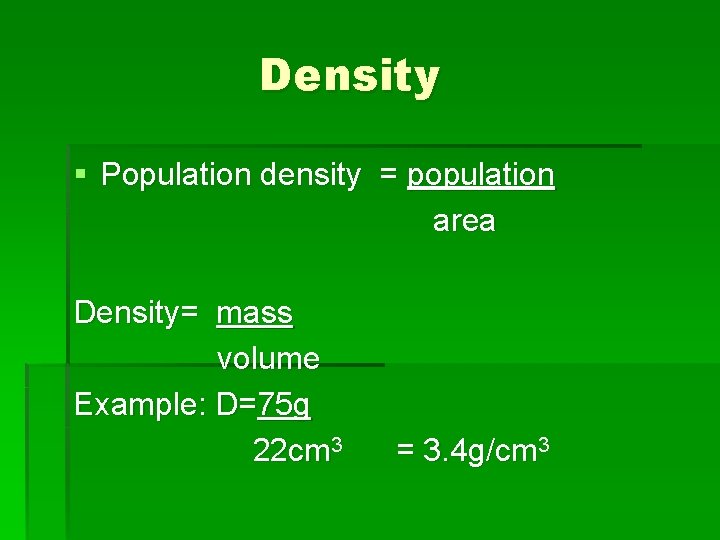
Density § Population density = population area Density= mass volume Example: D=75 g 22 cm 3 = 3. 4 g/cm 3

Significant Digits § Significant Digits: The certain digits and the estimated digits of a measurement are together.

More on Significant Digits § Atlantic Pacific Rule: A rule that can help you identify the significant digits in a measurement. A) Decimal present, count from the Pacific side. B) First nonzero digit C) All other digits are significant.

Atlantic Pacific Rule § 0. 0093077

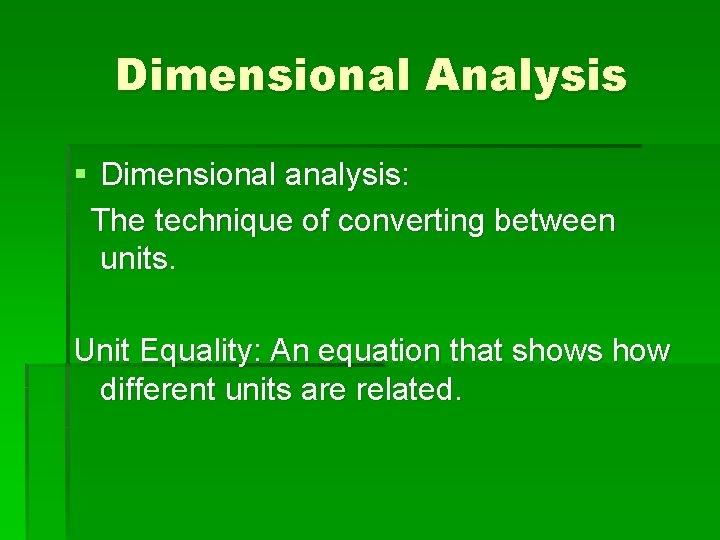
Dimensional Analysis § Dimensional analysis: The technique of converting between units. Unit Equality: An equation that shows how different units are related.

Conversion Factors § Conversion factors: Fraction having a value of one that is written from a unit equality and is used to change a measurement from one unit to another.

More on Conversions § How to do conversions: 1 gal = 3. 785 L 1 gal 1 = 3. 78 L 1 gal (Cross Multiply)

Problem Solving § 4 step problem solving strategy: 1) Analyze 2) Plan 3) Solve 4) Evaluate

Graphing § Graphing: 1) label each axis with name and variable in units of measurements 2) Make a title 3) Plot the points 4) connect the data points with the best fit line

Graphing

Ways of Showing Data