What is an ideal drug In the early





































- Slides: 41


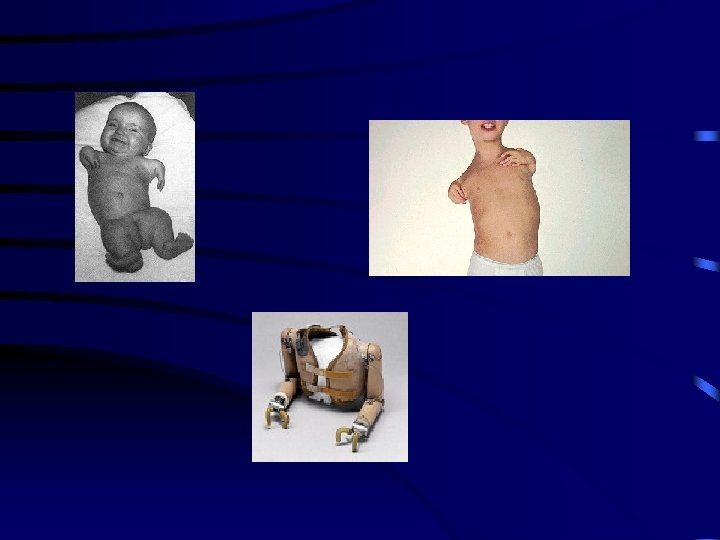
What is an ideal drug? • In the early 1900 s Paul Ehrlich described an ideal drug as a magic bullet. Such a drug would be aimed precisely at a disease site and would not harm healthy tissues.

Risk • No medicinal product is entirely or absolutely safe for all people, in all places, at all times. We must always live with some measure of uncertainty. 3/6/2021

We Must Always Ask: • Do the potential benefits outweigh the potential risks for this individual? ACHP

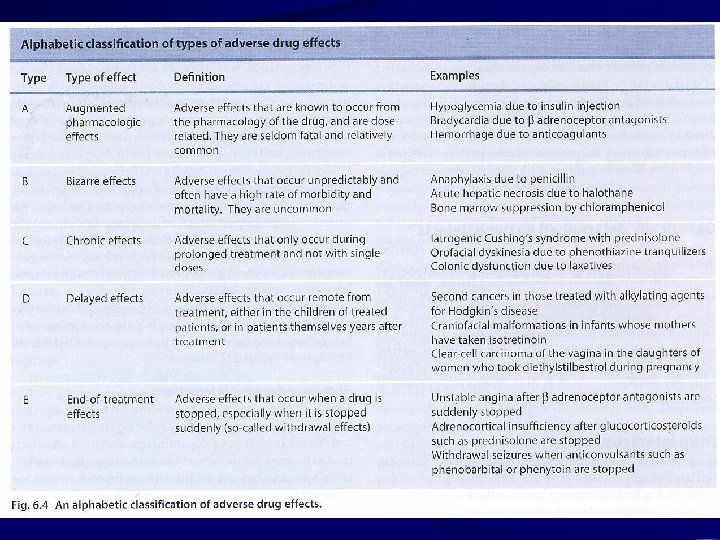
Adverse Drug Reaction WHO definition: Any response to a drug which is Noxious and Unintended, and which occurs at doses used in man for prophylaxis, diagnosis or treatment.


History of drug safety after thalidomide eradication Ø 1961 : Dr William Mc. Bride (Australia)( thalidomide 4000 cases) Ø 1964 : UK started “yellow cards” system Ø 1968 : start of WHO Programme for International Drug Monitoring


Why Should We Learn about Adverse Drug Reactions (ADR)? § Over 2 MILLION serious ADRs yearly § 100, 000 DEATHS yearly § 6. 7% of hospitalized patients have an ADR with a fatality of 0. 32, Ref: U. S. Food and Drug Administration. Center for Drug Evaluation and Research

Costs Associated with ADRs Ø $ 136 BILLION yearly (related to morbidity and mortality) Ø Greater than total costs of cardiovascular or diabetic care. Ø Mean length of stay, cost and mortality ADR patients are DOUBLE that for control group of patients without ADR. Ø ADRs cause 1 out of 5 injuries or deaths per year to hospitalized patients. Ref: U. S. Food and Drug Administration. Center for Drug Evaluation and Research

ADR has financial and social effects: 1 - Unreliability on manufacturer 2 - Unreliability on health system (Physician, Pharmacist & Nurse) 3 - Unreliability on governments in saving the social safety 4 - Causing mortality & morbidity

So many prescriptions!

§ § § Tow-thirds of patients visits result in a prescription 2. 8 BILLION outpatients prescriptions were filled in the year 2000 (about 10 prescriptions person in the U. S. ) ADRs increase exponentially with 4 or more medications Ref: U. S. Food and Drug Administration. Center for Drug Evaluation and Research

Who might get an ADR? • Anyone who takes a medicine – Differential diagnosis should include the possibility of an ADR if the patient is taking any form of medication

Who is most at risk from ADRs? Patients who; • are young, or old or female • are taking multiple therapies – 50% of patients on 5 drugs or more • have more than one medical problem • have a history of allergy or a previous reaction to drugs

Older Adults and Medications • Older adults make up 13% of population • Account for: – About 30% of prescribed medications – About 40% of over-the-counter medications • At least 90% take at least one prescription medication • 12% use ten or more per week ACHP


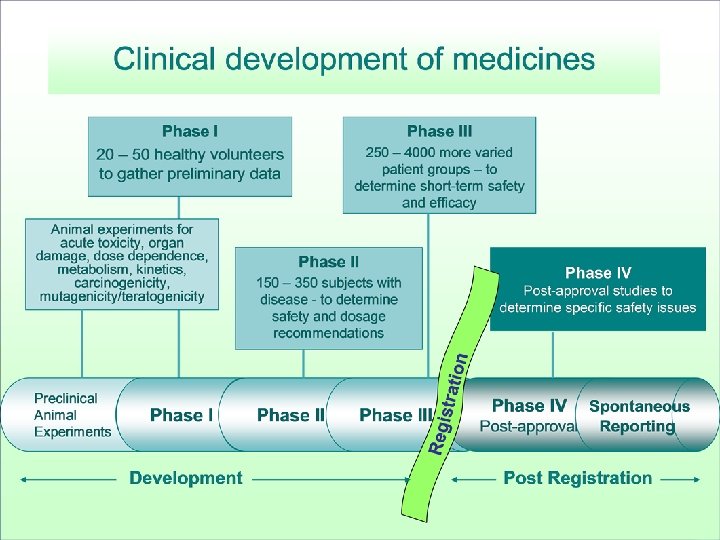
How Knowledge About ADRs Is Created? 1 -Animal experiments 2 - Clinical trials 3 - Epidemiological methods Ø Spontaneous reporting Ø Cohort studies Ø Case-control studies

Limitations of Clinical Trials Limited size Ø Narrow population Ø Narrow indications Ø Short duration Ø • Ref: J. Russell May. Adverse drug Reactions and interaction, In: Pharmacotherapy, A pathophysiologic Approach. 1997, Appleton & Lange.

Drug Development

How many patients one needs to treat to see with high probability the reaction? Ø Pre-marketing studies are carried out in limited number of patients: “The law of three” – In order to detect for sure SAE that occurs as 1 event per 2000 patients treated we need to treat • 6000 patients • 9600 patients • 13000 patients for 1 case for 2 cases for 3 cases • The number of patients involved in pre-marketing studies has been increasing but is still limited in comparison with the exposure to the drug in post-marketing phase

Some drugs cause serious ADRs at very low frequencies Ø bromfenac hepatotoxicity 1 in 20, 000 patients, removed from the market in 1998, less than 1 year after it was introduced). • Ref: U. S. Food and Drug Administration. Center for Drug Evaluation and Research

Ø Assessment the quality of medications Ø Assessment of drug safety Ø Detection of occurrence rate of ADR Ø Decreasing the risk of occurrence of adverse events

Spontaneous Reporting Large population All medicines Hospital and out-patient care Long perspective Patient analysis possible Non-interventional Cheap

Examples of product recalls due to toxicity • Medicine • Thalidomide 1965 • Practolol • Clioquinol • Benoxaprofen 1982 • Terfenadine • Rofecoxib • Veralipride Year 1975 1970 1997 2004 2007 • Examples of serious and unexpected adverse events leading to withdrawal of medicine • Phocomelia • Sclerosing peritonitis • Subacute nephropathy • Nephrotoxicity, cholestatic jaundice • Torsade de pointes • Cardiovascular effects • Anxiety, depression, movement disorders

Pharmaco - Vigilance • Pharmaco = medicine • Vigilare = to watch – alert watchfulness – forbearance of sleep; wakefulness – watchfulness in respect of danger; care; caution; circumspection – the process of paying close and continuous attention

Detection, Assessment & Prevention of ADRs in Human. Ref: World Health Organization.


Pharmacovigilance Major Aims ü Early detection of unknown reactions and interactions ü Detection of increase in frequency ü Identification of risk factors ü Quantifying risks ü Preventing patients from being affected unnecessarily ü RATIONAL AND SAFE USE OF DRUGS Ref: World Health organization.

The ultimate goal of pharmacovigilance is improving pharmacotherapy Ref: World Health Organization

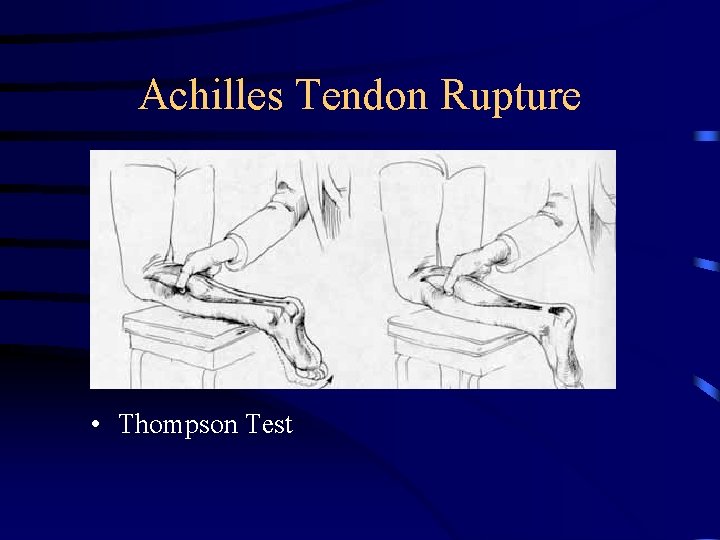
Case #11: 34 yo female with DM, Hypertension, depression and recent UTI, taking metformin, insulin, lisinopril, paxil and ciprofloxacin. Presents with severe calf pain and difficulty with plantar flexion What is the most likely cause? A) Peroneal Nerve Palsy B) Diabetic Neuropathy C) Transient ischemia event D) Achilles Tendon Rupture

Case #11 (cont). Which medication is the most likely cause? A) B) C) D) E) Metformin Lisinopril Paxil Ciprofloxacin Insulin

Achilles Tendon Rupture • Thompson Test

Misconceptions about ADR Reporting Ø All serious ADRs are documented by the time a drug is marketed Ø About patient receiving multiple medications, it is difficult to determine if a drug is responsible for the ADR Ø ADRs should only be reported if absolutely certain Ø One reported case can’t make a different § Ref: U. S. Food and Drug Administration. Center for Drug Evaluation and Research

Countries with the best reporting rates generate: • Over 200 reports per 1, 000 inhibitants per year. • Over 150 reports per 1000 physicians per year.

International Vigilance Every healthcare professional in the world should be constantly alert for adverse effects or potentional new hazards and reporting them to their National Centers.



SEND A REPORT SAVE A LIFE Dr. Goodarzian

Med. Watch The FDA Safety Information and Adverse Event Reporting Program: www. fda. gov/medwatch/ Safety alerts Recalls Withdrawals Important labeling changes Biologicals, Drugs, Dietary supplements
