What is an Atom CNotes November 14 2012

What is an Atom? C-Notes November 14, 2012 8 th Grade Physical Science Ms. Mc. Kinley

Let’s Brainstorm… • Make a list of items that have a center and an outside that are made of different materials.

What did we come up with? ?
What is an Atom? • • • The smallest particle of an element The basic building blocks of matter The arrangement and types of atoms give matter its properties. Atoms are like legos! They are building blocks

What is an Atom? (cont’d) • 2000 years ago the Greeks defined atoms as the smallest parts of matter. • Greek word “atomos” means uncuttable
The Structure of an Atom • Two major components: – – • The NUCLEUS The ELECTRON CLOUD 3 basic subatomic particles (“subatomic” means smaller than an atom): – Protons – Neutrons – Electrons
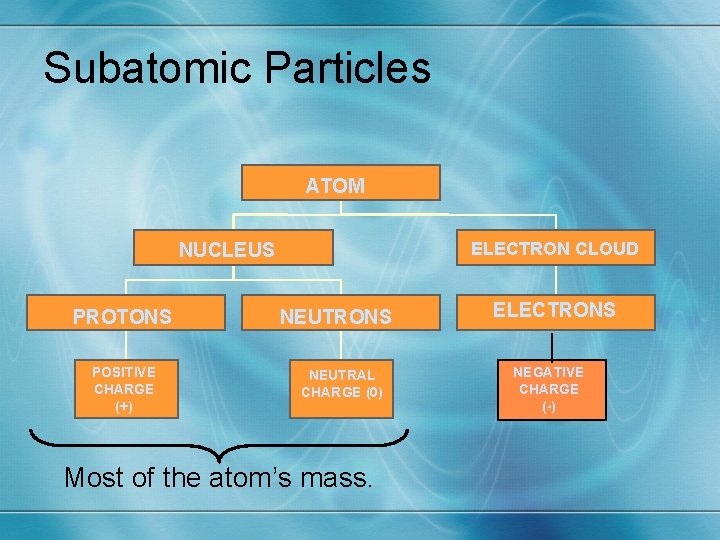
Subatomic Particles ATOM NUCLEUS ELECTRONS ELECTRON CLOUD PROTONS NEUTRONS ELECTRONS NEGATIVE CHARGE POSITIVE CHARGE (+) NEUTRAL CHARGE (0) CHARGE NEGATIVE CHARGE (-) Most of the atom’s mass.

The Nucleus • Protons and Neutrons combine to make the nucleus • Held in place by the Strong Force
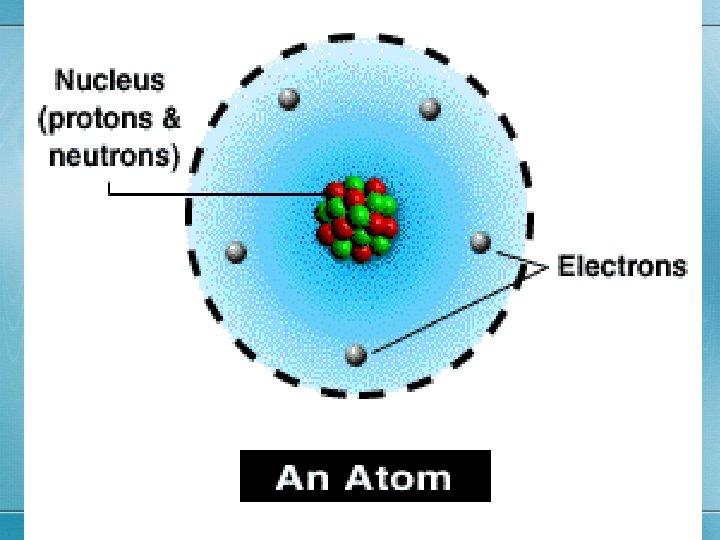
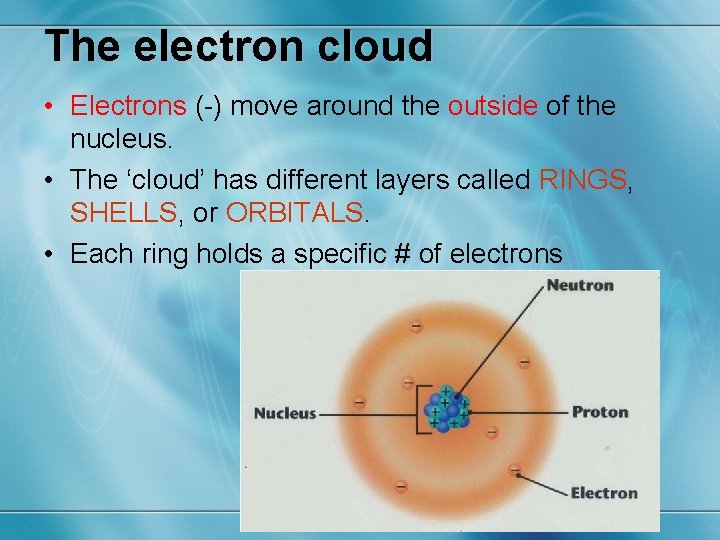
The electron cloud • Electrons (-) move around the outside of the nucleus. • The ‘cloud’ has different layers called RINGS, SHELLS, or ORBITALS. • Each ring holds a specific # of electrons
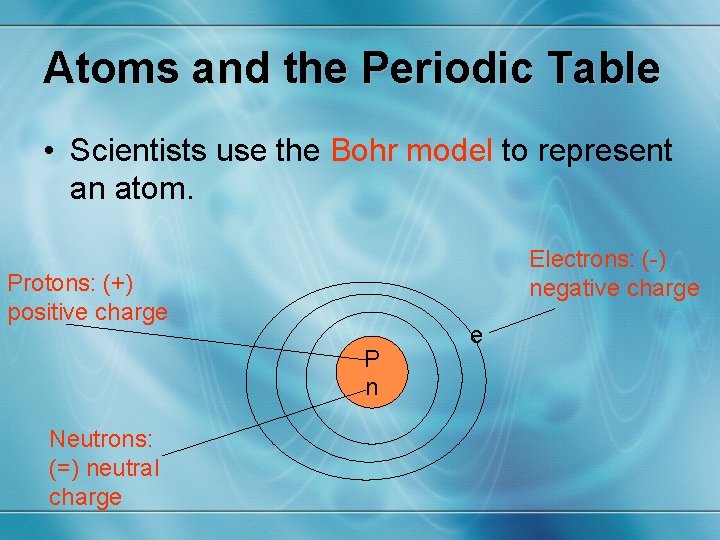
Atoms and the Periodic Table • Scientists use the Bohr model to represent an atom. Electrons: (-) negative charge Protons: (+) positive charge P n Neutrons: (=) neutral charge e
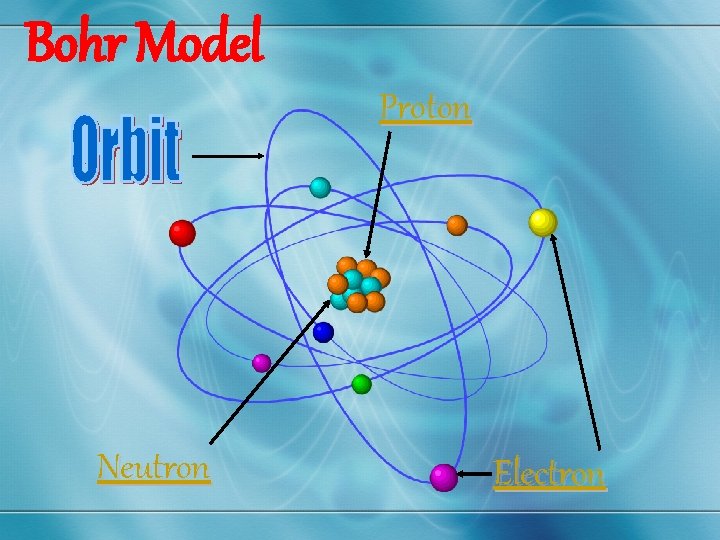
Bohr Model Proton Neutron Electron
- Slides: 12