What is an Acid A solution in which

- Slides: 8

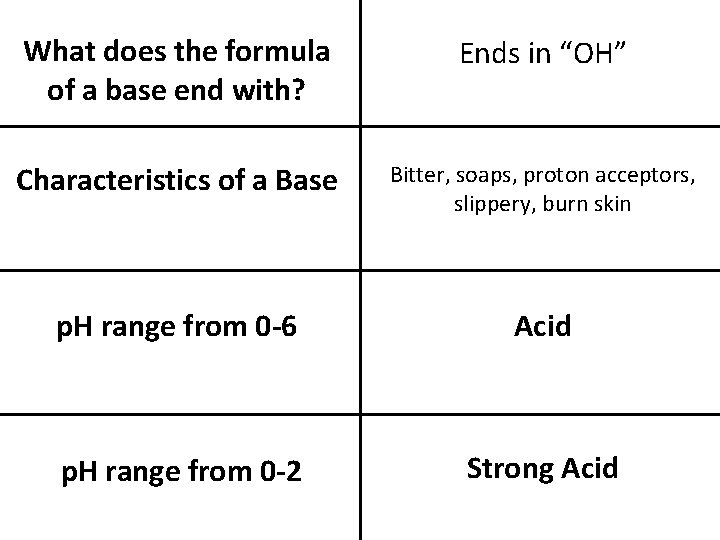
What is an Acid A solution in which hydronium ions are present when mixed with water What does the formula for an acid start with? Starts with an “H” Characteristics of an ACID Sour, Reacts with metals, electrolyte, burns the skins What is a Base A solution in which hydroxide ions are present

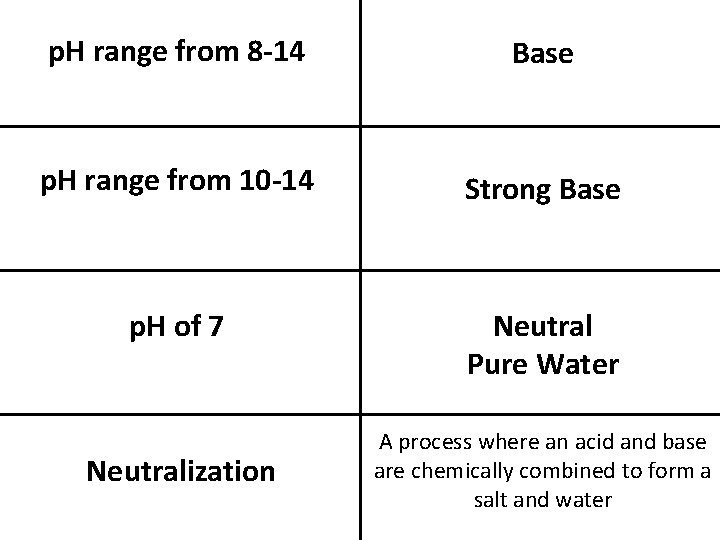
What does the formula of a base end with? Ends in “OH” Characteristics of a Base Bitter, soaps, proton acceptors, slippery, burn skin p. H range from 0 -6 Acid p. H range from 0 -2 Strong Acid

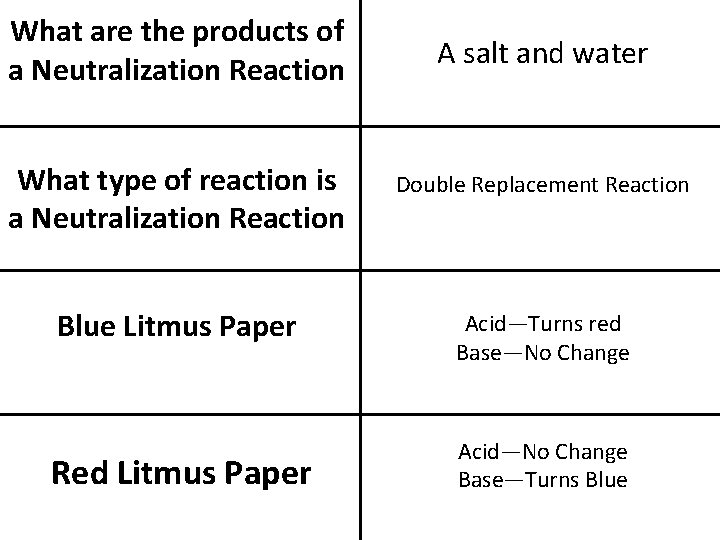
p. H range from 8 -14 Base p. H range from 10 -14 Strong Base p. H of 7 Neutral Pure Water Neutralization A process where an acid and base are chemically combined to form a salt and water

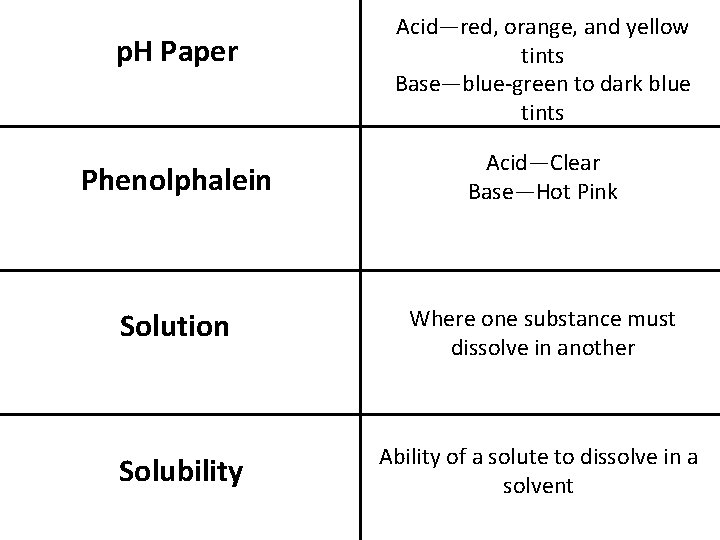
What are the products of a Neutralization Reaction A salt and water What type of reaction is a Neutralization Reaction Double Replacement Reaction Blue Litmus Paper Acid—Turns red Base—No Change Red Litmus Paper Acid—No Change Base—Turns Blue

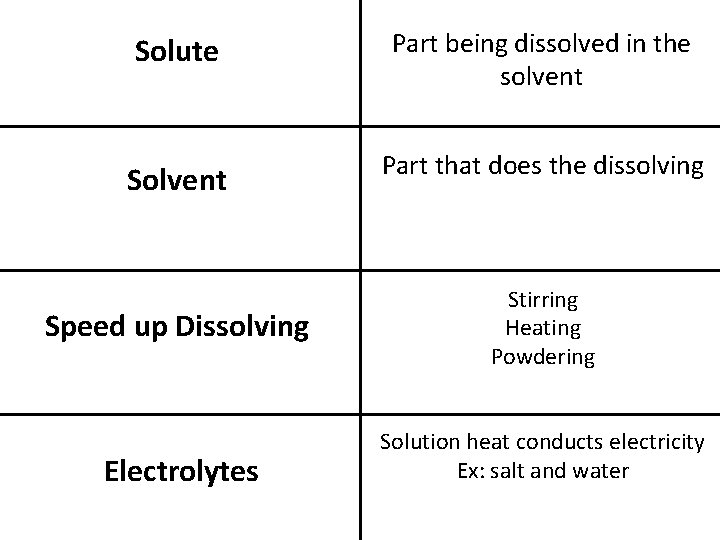
p. H Paper Acid—red, orange, and yellow tints Base—blue-green to dark blue tints Phenolphalein Acid—Clear Base—Hot Pink Solution Where one substance must dissolve in another Solubility Ability of a solute to dissolve in a solvent

Solute Part being dissolved in the solvent Solvent Part that does the dissolving Speed up Dissolving Electrolytes Stirring Heating Powdering Solution heat conducts electricity Ex: salt and water

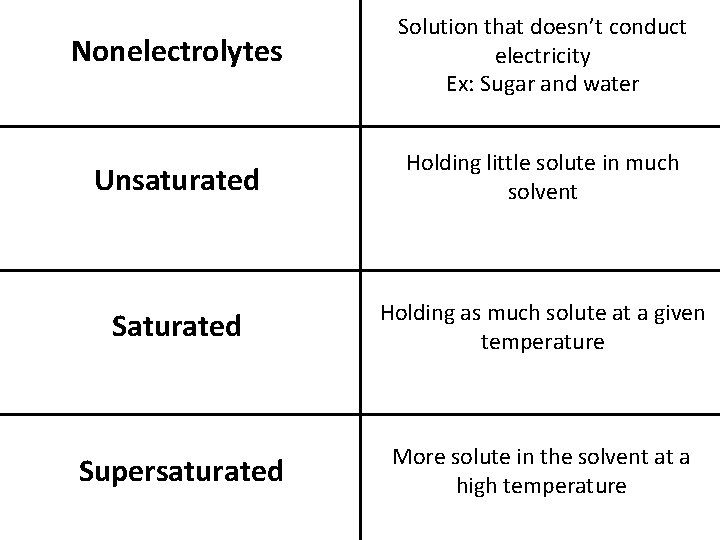
Nonelectrolytes Solution that doesn’t conduct electricity Ex: Sugar and water Unsaturated Holding little solute in much solvent Saturated Holding as much solute at a given temperature Supersaturated More solute in the solvent at a high temperature

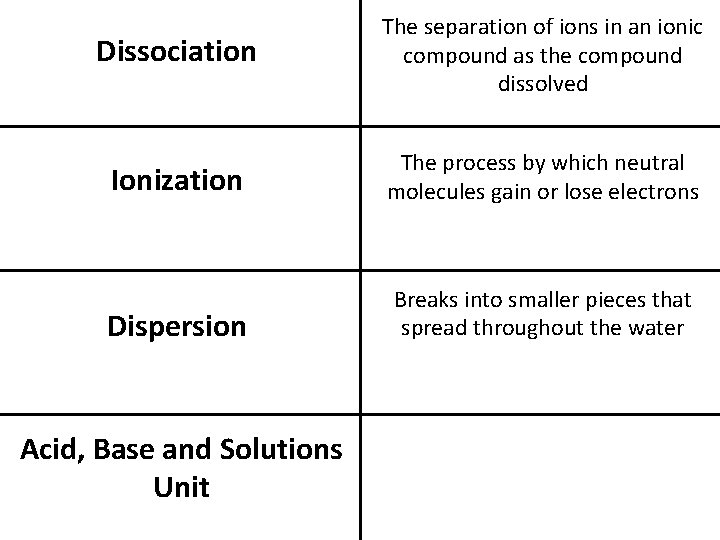
Dissociation The separation of ions in an ionic compound as the compound dissolved Ionization The process by which neutral molecules gain or lose electrons Dispersion Breaks into smaller pieces that spread throughout the water Acid, Base and Solutions Unit