What is an ABG Arterial Blood Gas Drawn
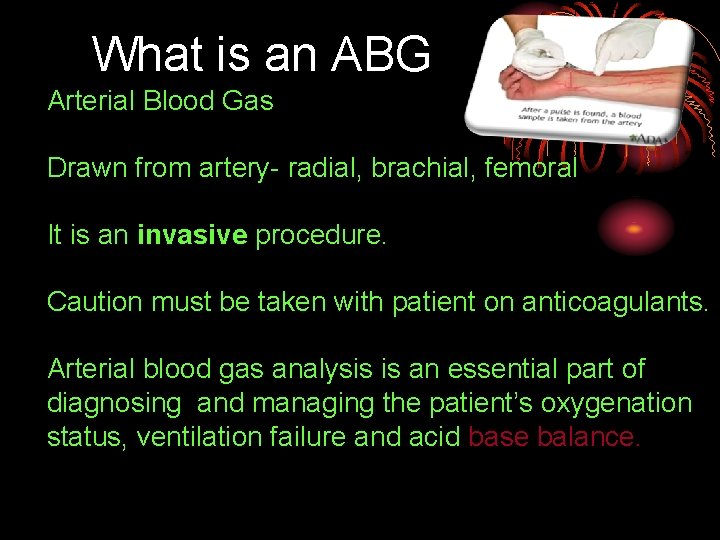
What is an ABG Arterial Blood Gas Drawn from artery- radial, brachial, femoral It is an invasive procedure. Caution must be taken with patient on anticoagulants. Arterial blood gas analysis is an essential part of diagnosing and managing the patient’s oxygenation status, ventilation failure and acid base balance.
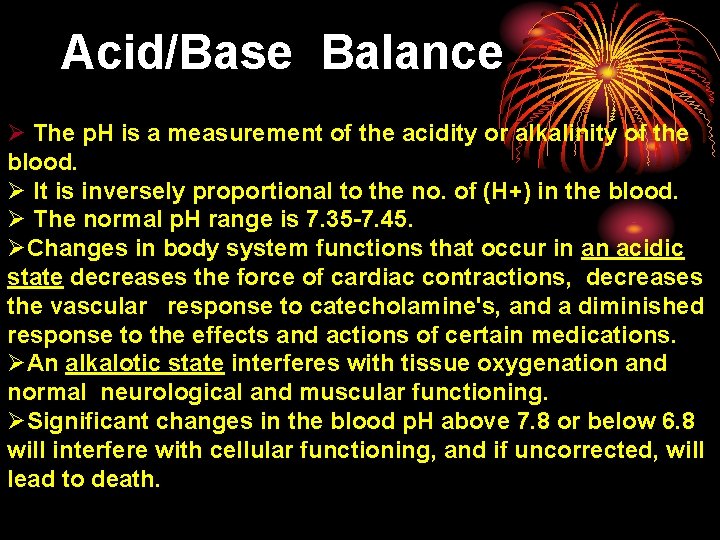
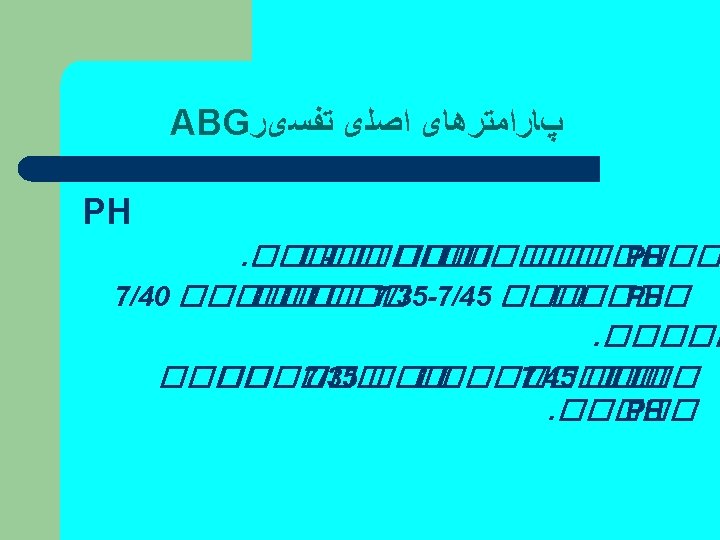
Acid/Base Balance Ø The p. H is a measurement of the acidity or alkalinity of the blood. Ø It is inversely proportional to the no. of (H+) in the blood. Ø The normal p. H range is 7. 35 -7. 45. ØChanges in body system functions that occur in an acidic state decreases the force of cardiac contractions, decreases the vascular response to catecholamine's, and a diminished response to the effects and actions of certain medications. ØAn alkalotic state interferes with tissue oxygenation and normal neurological and muscular functioning. ØSignificant changes in the blood p. H above 7. 8 or below 6. 8 will interfere with cellular functioning, and if uncorrected, will lead to death.
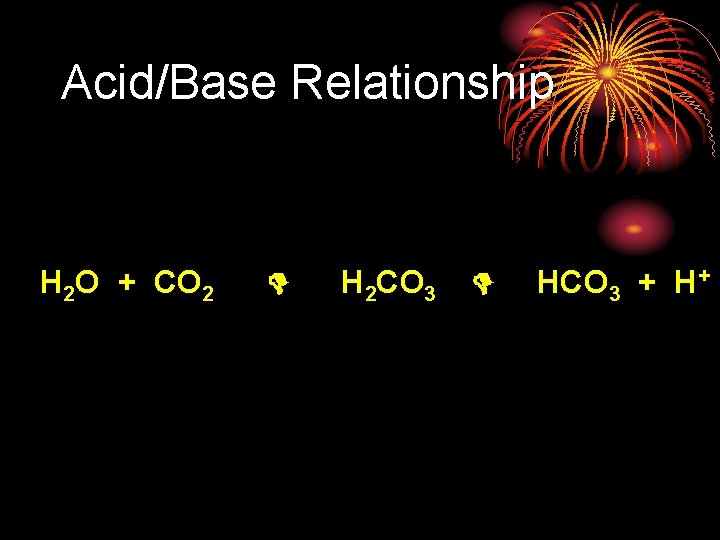
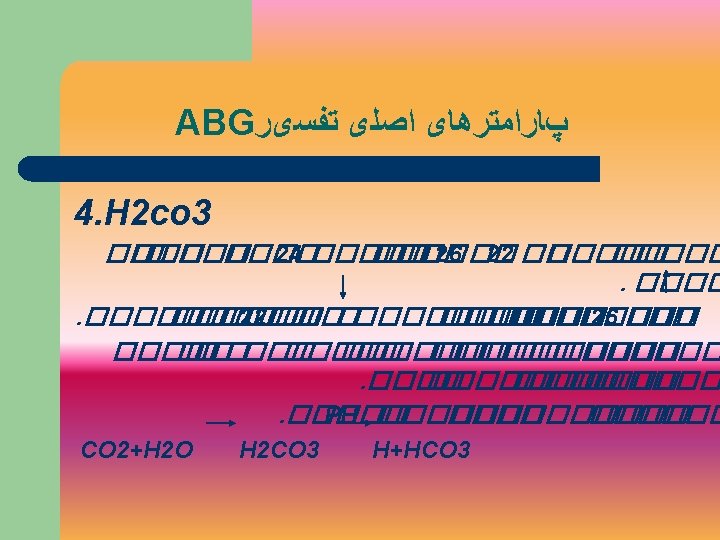
Acid/Base Relationship H 2 O + CO 2 H 2 CO 3 HCO 3 + H+

Buffers ØThere are two buffers that work in pairs ØH 2 CO 3 Na. HCO 3 Carbonic acid base bicarbonate ØThese buffers are linked to the respiratory and renal compensatory system

The Respiratory buffer response • The blood p. H will change acc. to the level of H 2 CO 3 present. • This triggers the lungs to either increase or decrease the rate and depth of ventilation • Activation of the lungs to compensate for an imbalance starts to occur within 1 -3 minutes






ACID BASE DISORDER Res. Acidosis • is defined as a p. H less than 7. 35 with a paco 2 greater than 45 mm. Hg. • Acidosis –accumulation of co 2, combines with water in the body to produce carbonic acid, thus lowering the p. H of the blood. • Any condition that results in hypoventilation cause respiratory acidosis.

Causes RES. ACIDOSIS 1. 2. 3. 4. 5. Central nervous system depression r/t medications such as narcotics, sedatives, or anesthesia. Impaired muscle function r/t spinal cord injury, neuromuscular diseases, or neuromuscular blocking drugs. Pulmonary disorders such as atelectasis, pneumonia, pneumothorax, pulmonary edema or bronchial obstruction Massive pulmonary embolus Hypoventilation due to pain chest wall injury, or abdominal pain.

Signs & symptoms of Respiratory Acidosis • Respiratory : Dyspn ea, respiratory distress and/or shallow respiration. • Nervous: Headache, restlessness and confusion. If co 2 level extremely high drowsiness and unresponsiveness may be noted. • CVS: Tachycardia and dysrhythmias

Management Res Acidosis • Increase the ventilation. • Causes can be treated rapidly include pneumothorax, pain and CNS depression r/t medication. • If the cause can not be readily resolved, mechanical ventilation.


Respiratory alkalosis • Psychological responses, anxiety or fear. • Pain • Increased metabolic demands such as fever, sepsis, pregnancy or thyrotoxicosis. • Medications such as respiratory stimulants. • Central nervous system lesions

Signs & symptoms Res Alkalosis • CNS: Light Headedness, numbness, tingling, confusion, inability to concentrate and blurred vision. • Dysrhythmias and palpitations • Dry mouth, diaphoresis and tetanic spasms of the arms and legs.

Management Res Alkalosis • Resolve the underlying problem • Monitor for respiratory muscle fatigue • When the respiratory muscle become exhausted, acute respiratory failure may ensue



Metabolic Acidosis • Bicarbonate less than 22 m. Eq/L with a p. H of less than 7. 35. • Renal failure • Diabetic ketoacidosis • Anaerobic metabolism • Starvation • Salicylate intoxication

Sign & symptoms Metabolic Acidosis • CNS: Headache, confusion and restlessness progressing to lethargy, then stupor or coma. • CVS: Dysrhythmias • Kussmaul’s respirations • Warm, flushed skin as well as nausea and vomiting

Management Metabolic Acidosis • Treat the cause • Hypoxia of any tissue bed will produce metabolic acids as a result of anaerobic metabolism even if the pao 2 is normal • Restore tissue perfusion to the hypoxic tissues • The use of bicarbonate is indicated for known bicarbonate - responsive acidosis such as seen with renal failure


Metabolic alkalosis • Bicarbonate more than 26 m Eq /L with a p. H more than 7. 45 • Excess of base /loss of acid can cause • Ingestion of excess antacids, excess use of bicarbonate, or use of lactate in dialysis. • Protracted vomiting, gastric suction, hypchoremia, excess use of diuretics, or high levels of aldesterone.

Signs/symptoms Metabolic Alkalosis • CNS: Dizziness, lethargy disorientation, siezures & coma. • M/S: weakness, muscle twitching, muscle cramps and tetany. • Nausea, vomiting and respiratory depression. • It is difficult to treat.

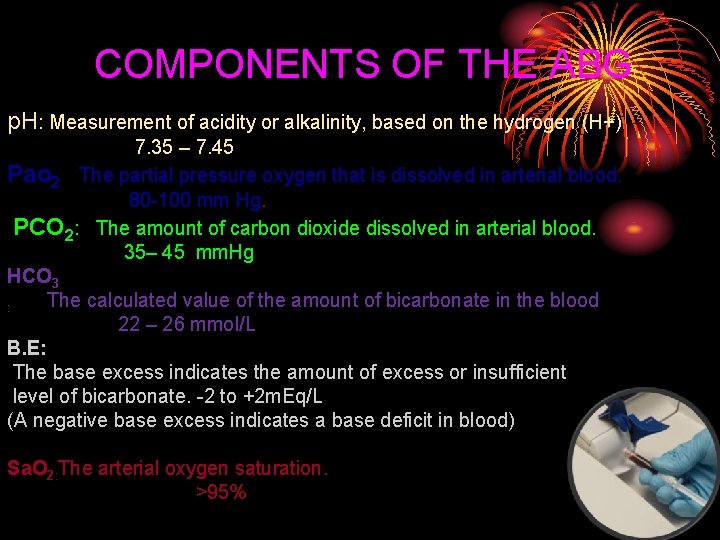
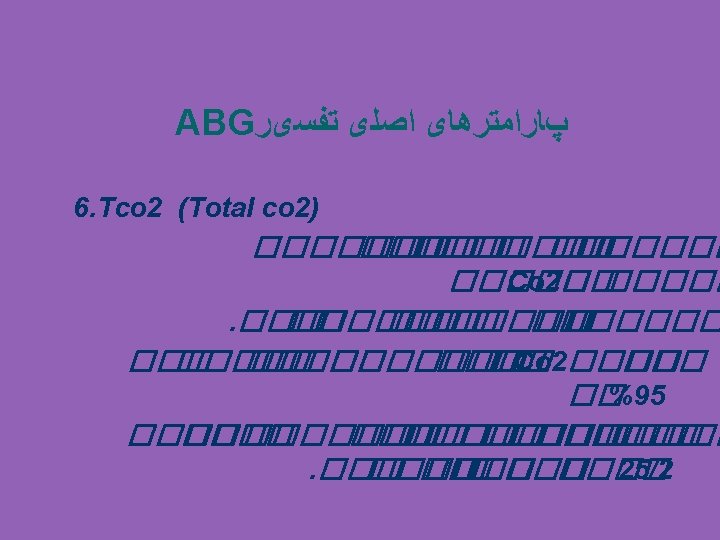
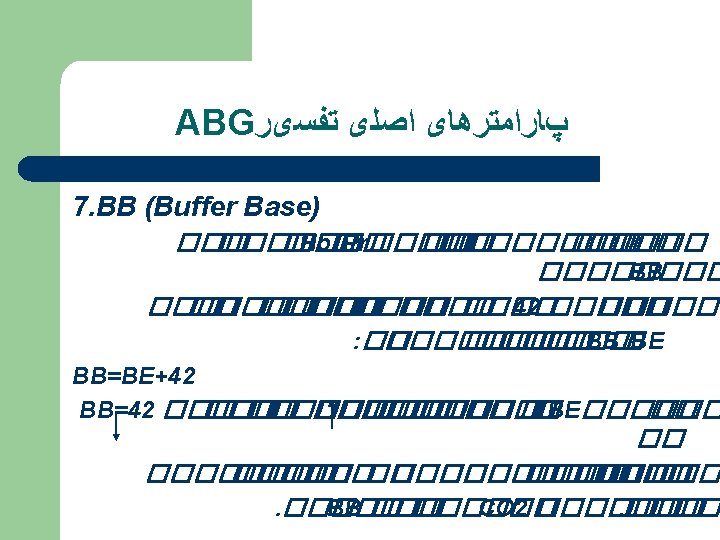
COMPONENTS OF THE ABG p. H: Measurement of acidity or alkalinity, based on the hydrogen (H+) 7. 35 – 7. 45 Pao 2 The partial pressure oxygen that is dissolved in arterial blood. 80 -100 mm Hg. PCO 2: The amount of carbon dioxide dissolved in arterial blood. 35– 45 mm. Hg HCO 3 The calculated value of the amount of bicarbonate in the blood : 22 – 26 mmol/L B. E: The base excess indicates the amount of excess or insufficient level of bicarbonate. -2 to +2 m. Eq/L (A negative base excess indicates a base deficit in blood) Sa. O 2: The arterial oxygen saturation. >95%




Stepwise approach to ABG • Step 1: Acidemic or Alkalemic? • Step 2: Is the primary disturbance respiratory or metabolic? • Step 3. Asses to Pa O 2. A value below 80 mm Hg indicates Hypoxemia. For a respiratory disturbance, determine whether it is acute or chronic. • Step 4. For a metabolic acidosis, determine whether an anion gap is present. • Step 5. Assess the normal compensation by the respiratory system for a metabolic disturbance

STEPS TO AN ABG INTERPRETATION • • Step: 1 Assess the p. H –acidotic/alkalotic If above 7. 5 – alkalotic If below 7. 35 – acidotic

Step 2 • Assess the pa. CO 2 level. • p. H decreases below 7. 35, the pa. CO 2 should rise. • If p. H rises above 7. 45 pa. CO 2 should fall. • If p. H and pa. CO 2 moves in opposite direction – primary respiratory problem.

Step 2 • Assess HCO 3 value • If p. H increases the HCO 3 should also increase • If p. H decreases HCO 3 should also decrease • They are moving in the same direction • primary problem is metabolic

Step 3 Assess pao 2 < 80 mm Hg - Hypoxemia For a resp. disturbance : acute or chronic v The differentiation between A/C & CHR. respiratory disorders is based on whethere is associated acidemia / alkalemia. v If the change in paco 2 is associated with the change in p. H, the disorder is acute. v In chronic process the compensatory process brings the p. H to within the clinically acceptable range ( 7. 30 – 7. 50)
Example 1 • J is a 45 years old female admitted with the severe attack of asthma. She has been experiencing increasing shortness of breath since admission three hours ago. Her arterial blood gas result is as follows: • p. H : 7. 22 • pa. CO 2 : 55 • HCO 3 : 25 • Follow the steps • p. H is low – acidosis • pa. CO 2 is high – in the opposite direction of the p. H. • Hco 3 is Normal. • Respiratory Acidosis • Need to improve ventilation by oxygen therapy, mechanical ventilation, pulmonary toilet or by administering bronchodilators.

EXAMPLE 2 • Mr. D is a 55 years old admitted with recurring bowel obstruction has been experiencing intractable vomiting for the last several hours. His ABG is: • p. H : 7. 5 • pa. CO 2 : 42 • HCO 3 : 33 • Metabolic alkalosis • Management: IV fluids, measures to reduce the excess base
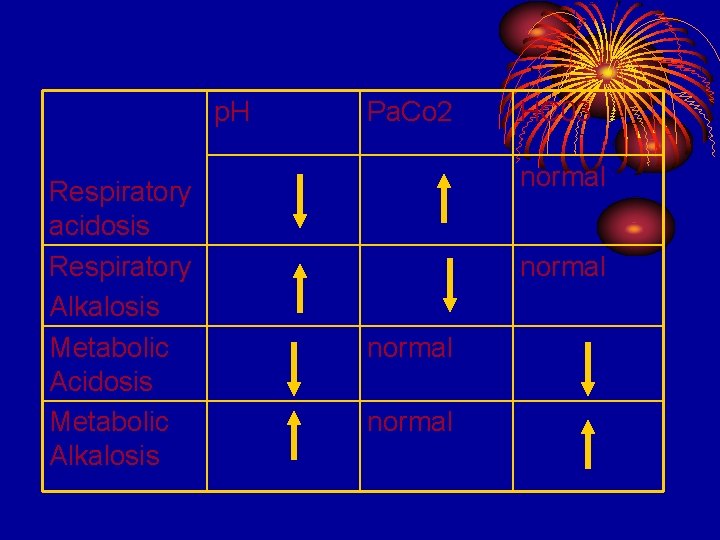
p. H Respiratory acidosis Respiratory Alkalosis Metabolic Acidosis Metabolic Alkalosis Pa. Co 2 HC 03 normal

BASE EXCESS • Is a calculated value estimates the metabolic component of an acid based abnormality. • It is an estimate of the amount of strong acid or base needed to correct the met. component of an acid base disorder (restore plasma p. H to 7. 40 at a Paco 2 40 mm. Hg)

Formula • With the base excess is -10 in a 50 kg person with metabolic acidosis m. M of Hco 3 needed for correction is: = 0. 3 X body weight X BE = 0. 3 X 50 X 10 = 150 m. M
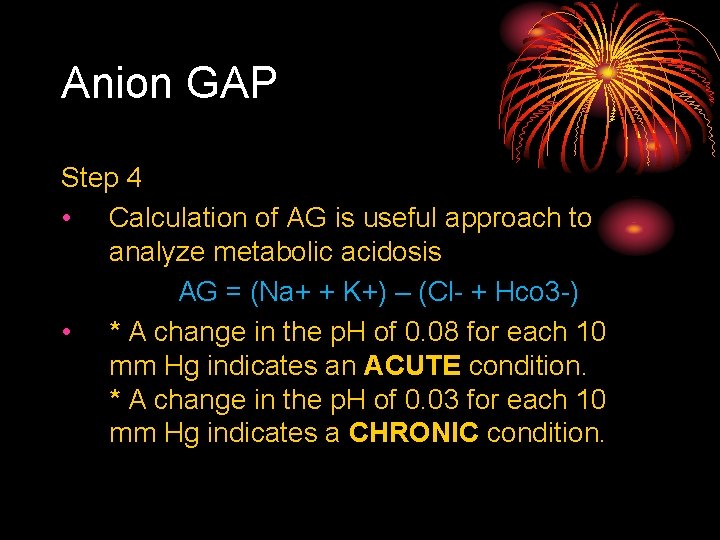
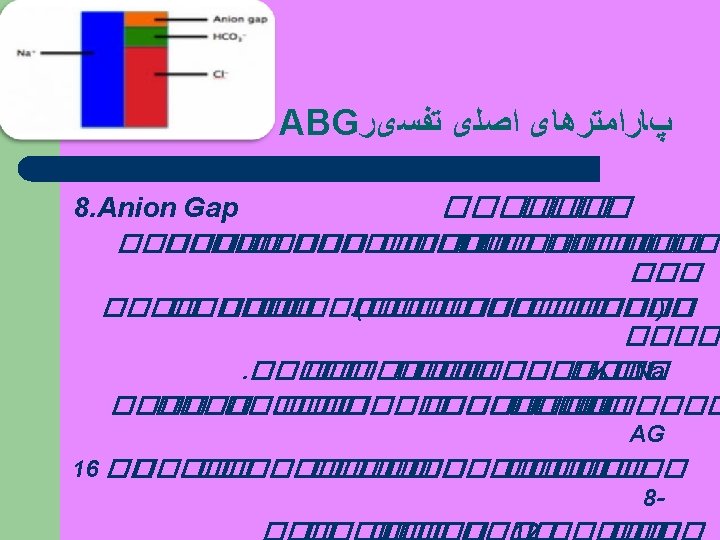
Anion GAP Step 4 • Calculation of AG is useful approach to analyze metabolic acidosis AG = (Na+ + K+) – (Cl- + Hco 3 -) • * A change in the p. H of 0. 08 for each 10 mm Hg indicates an ACUTE condition. * A change in the p. H of 0. 03 for each 10 mm Hg indicates a CHRONIC condition.

Step 5 • A patient can be uncompensated or partially compensated or fully compensated • p. H remains outside the normal range • p. H has returned within normal rangefully compensated though other values may be still abnormal • Be aware that neither the system has the ability to overcompensate

ABG Interpretation Step 5 cont… Determine if there is a compensatory mechanism working to try to correct the p. H. ie: if have primary respiratory acidosis will have increased Pa. CO 2 and decreased p. H. Compensation occurs when the kidneys retain HCO 3.

Assess the Pa. CO 2 • In an uncompensated state – when the p. H and pa. CO 2 moves in the same direction: the primary problem is metabolic. • The decreasing paco 2 indicates that the lungs acting as a buffer response (blowing of the excess CO 2) • If evidence of compensation is present but the p. H has not been corrected to within the normal range, this would be described as metabolic disorder with the partial respiratory compensation.

Assess the HCO 3 • The p. H and the HCO 3 moving in the opposite directions, we would conclude that the primary disorder is respiratory and the kidneys acting as a buffer response: are compensating by retaining HCO 3 to return the p. H to normal range.

Example 3 • Mrs. H is admitted, he is kidney dialysis patient who has missed his last 2 appointments at the dialysis centre his ABG results: • p. H : 7. 32 • pa. Co 2 : 32 • HCO 3 : 18 • Pao 2 : 88 • Partially compensated metabolic Acidosis
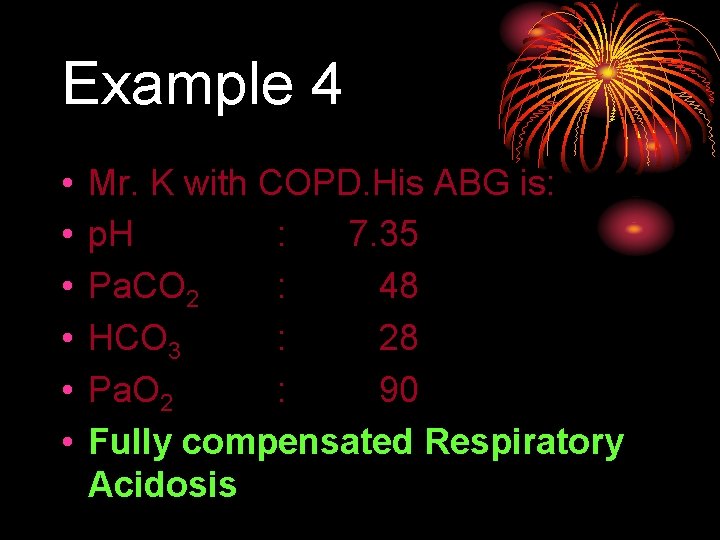
Example 4 • • • Mr. K with COPD. His ABG is: p. H : 7. 35 Pa. CO 2 : 48 HCO 3 : 28 Pa. O 2 : 90 Fully compensated Respiratory Acidosis

Example 5 • Mr. S is a 53 year old man presented to ED with the following ABG. • p. H : 7. 51 • Pa. CO 2 : 50 • HCO 3 : 40 • Pao 2 : 40 (21%O 2) • He has metabolic alkalosis • Acute respiratory alkalosis (acute hyperventilation).
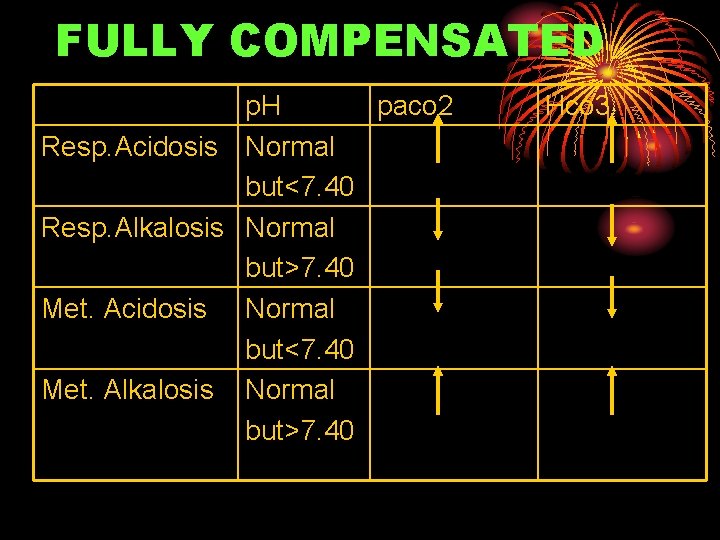
FULLY COMPENSATED p. H paco 2 Resp. Acidosis Normal but<7. 40 Resp. Alkalosis Normal but>7. 40 Met. Acidosis Normal but<7. 40 Met. Alkalosis Normal but>7. 40 Hco 3
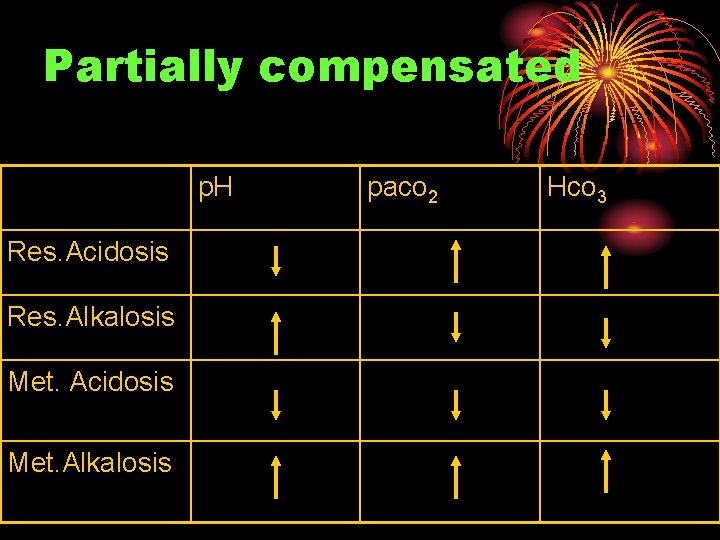
Partially compensated p. H Res. Acidosis Res. Alkalosis Met. Acidosis Met. Alkalosis paco 2 Hco 3

Precautions Ø Excessive Heparin Decreases bicarbonate and Pa. CO 2 Ø Large Air bubbles not expelled from sample Pa. O 2 rises, Pa. CO 2 may fall slightly. Ø Fever or Hypothermia, Hyperventilation or breath holding (Due to anxiety) may lead to erroneous lab results Ø Care must be taken to prevent bleeding
































Thanks for attention
- Slides: 102