What is Acid Rain Acid Rain in PA








- Slides: 16

What is Acid Rain ?



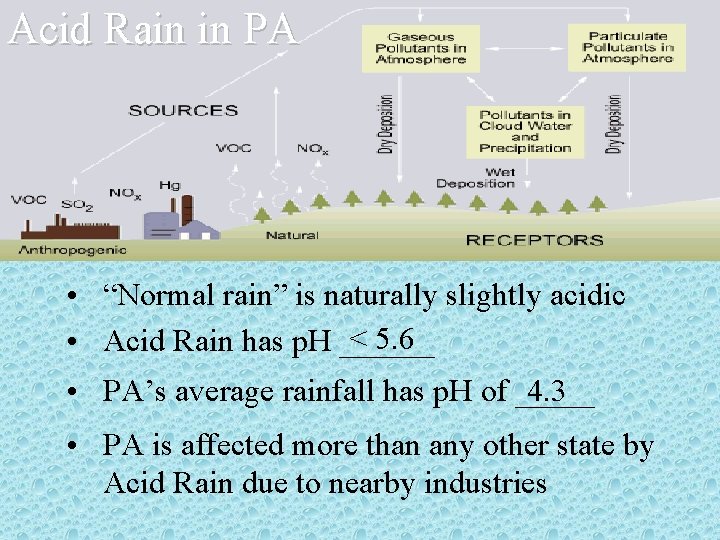
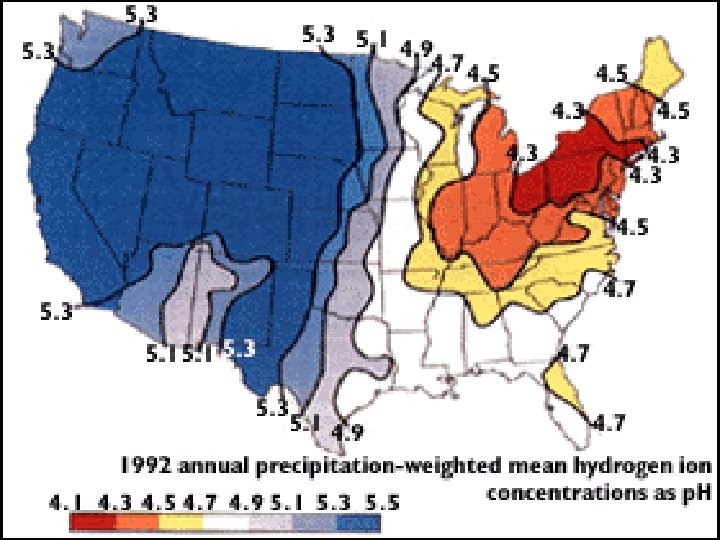
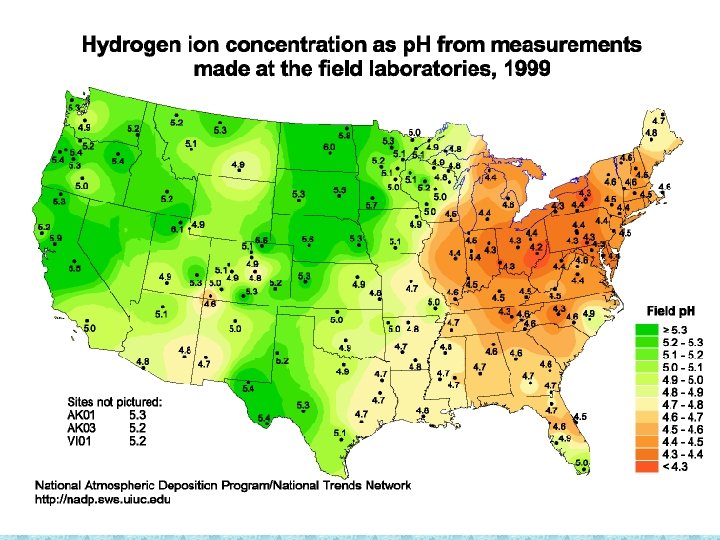
Acid Rain in PA • “Normal rain” is naturally slightly acidic < 5. 6 • Acid Rain has p. H ______ • PA’s average rainfall has p. H of _____ 4. 3 • PA is affected more than any other state by Acid Rain due to nearby industries

Acid Rain Formation • Burning fossil fuels (coal, oil, gas) • NOx and SOx gases are emitted • NOx and SOx combine with water in the sky to form nitric and sulfuric acid • NO 2 + H 2 O HNO 3 • SO 2 + H 2 O H 2 SO 4 Answers Question # 9 on Packet Page 6

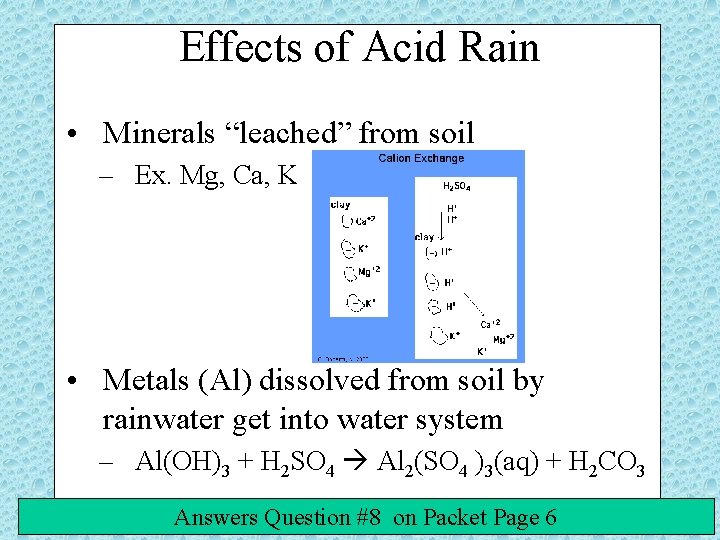
Effects of Acid Rain • Minerals “leached” from soil – Ex. Mg, Ca, K • Metals (Al) dissolved from soil by rainwater get into water system – Al(OH)3 + H 2 SO 4 Al 2(SO 4 )3(aq) + H 2 CO 3 Answers Question #8 on Packet Page 6

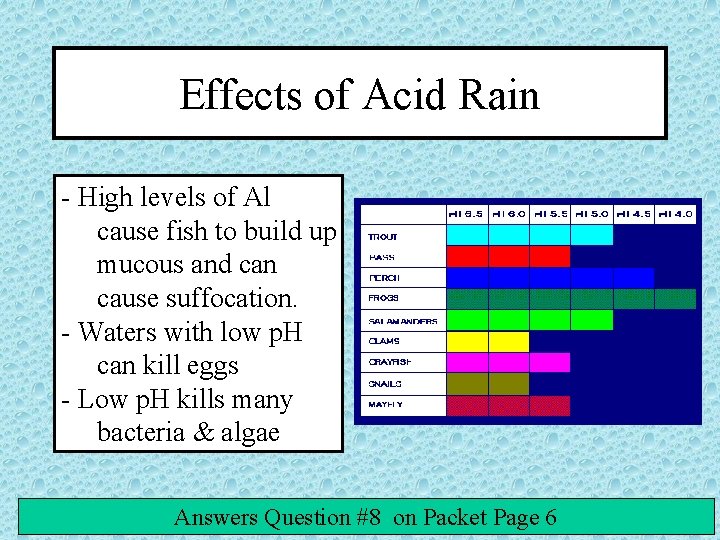
Effects of Acid Rain - High levels of Al cause fish to build up mucous and can cause suffocation. - Waters with low p. H can kill eggs - Low p. H kills many bacteria & algae Answers Question #8 on Packet Page 6

Effects of Acid Rain • Dissolves waxy coating on plants – leaves them vulnerable to fungus & bacteria • Dissolves Ca. CO 3 (marble, limestone, concrete) • Reacts with some metals Answers Question #8 on Packet Page 6

Effects of Acid Rain Since 1998, Harvard University wraps some of the bronze and marble statues on its campus, such as this “Chinese Stele", with waterproof covers every winter, in order to protect them from erosion caused by acid rain (or, actually, acid snow)

The effect of acid rain on statuary Location: New York City

A forest damaged by acid rain

Acid Rain: Interstate & International - In the past, factories had short funnels to let out smoke. - This caused local pollution. - Now, factories have taller smoke stacks. - This leads to interstate and international pollution. Answers Question #10 on Packet Page 7



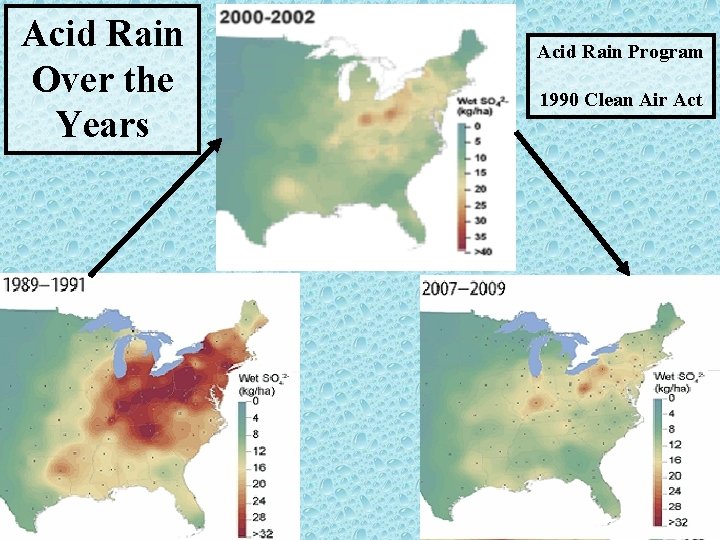
Acid Rain Over the Years Acid Rain Program 1990 Clean Air Act

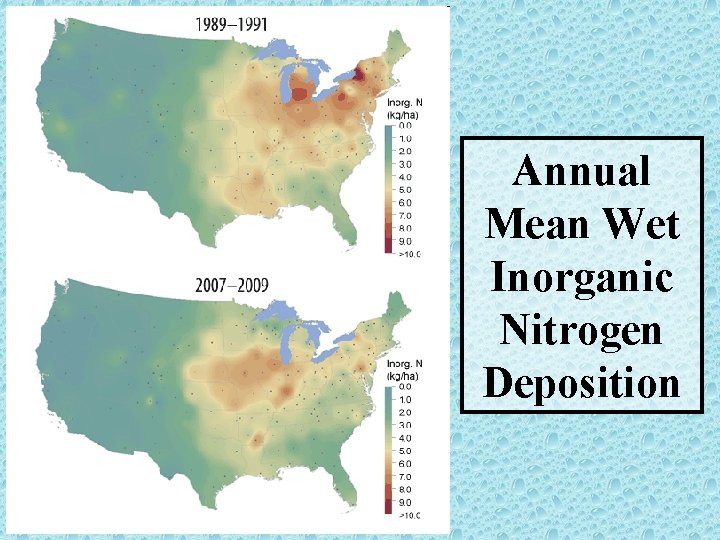
Annual Mean Wet Inorganic Nitrogen Deposition