What is a covalent bond types of elements

�What is a covalent bond? types of elements form covalent bonds?
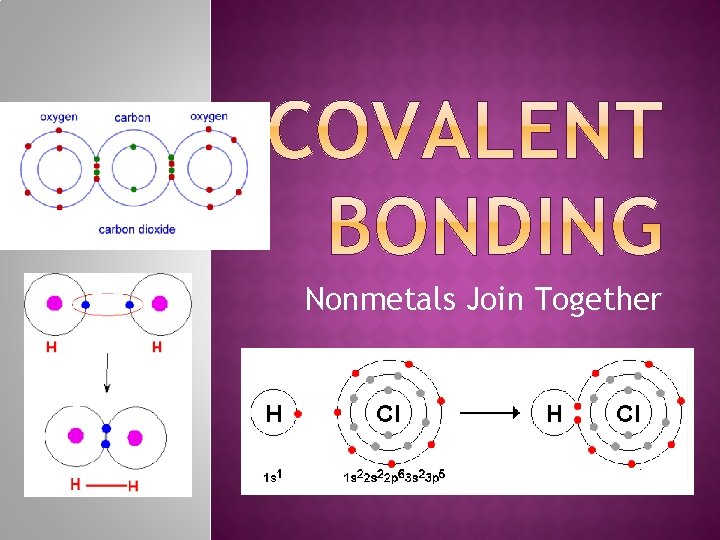
Nonmetals Join Together
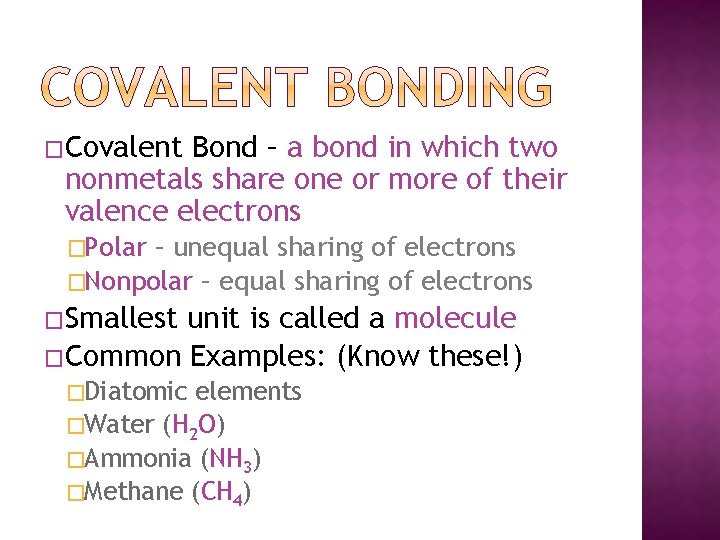
�Covalent Bond – a bond in which two nonmetals share one or more of their valence electrons �Polar – unequal sharing of electrons �Nonpolar – equal sharing of electrons �Smallest unit is called a molecule �Common Examples: (Know these!) �Diatomic elements �Water (H 2 O) �Ammonia (NH 3) �Methane (CH 4)
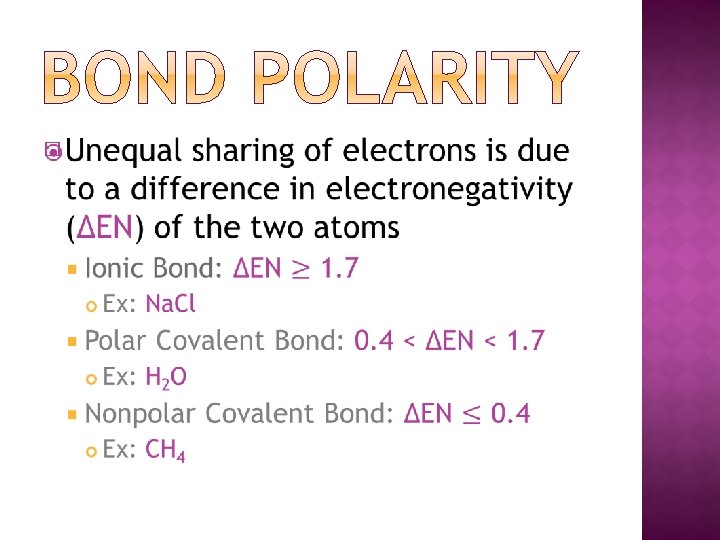
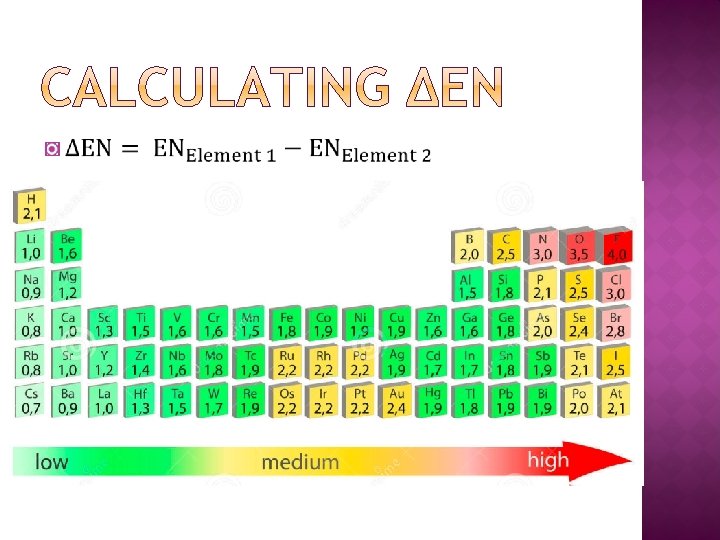
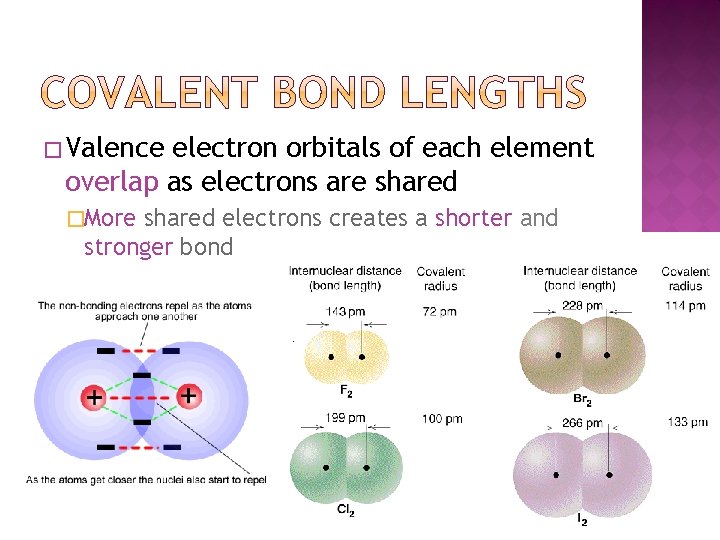
� Valence electron orbitals of each element overlap as electrons are shared �More shared electrons creates a shorter and stronger bond

�Low melting and boiling points �Many are liquids and gases at room temperature �Soluble in water IF they are polar (like dissolves like) �Do not conduct electricity
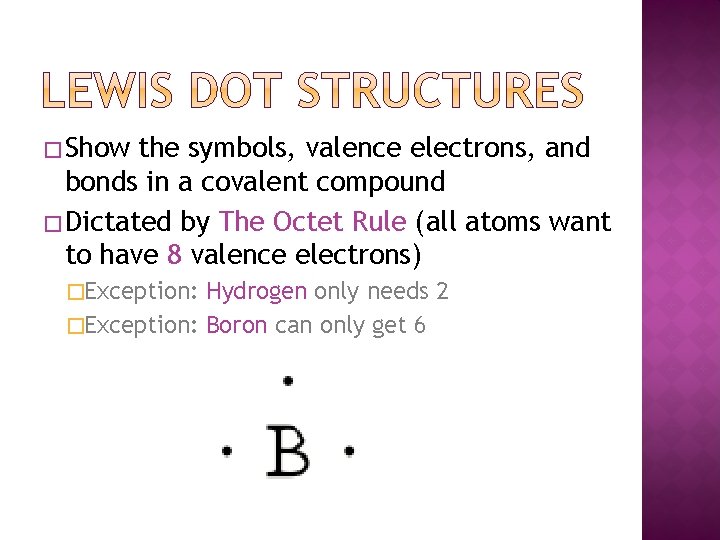
� Show the symbols, valence electrons, and bonds in a covalent compound � Dictated by The Octet Rule (all atoms want to have 8 valence electrons) �Exception: Hydrogen only needs 2 �Exception: Boron can only get 6
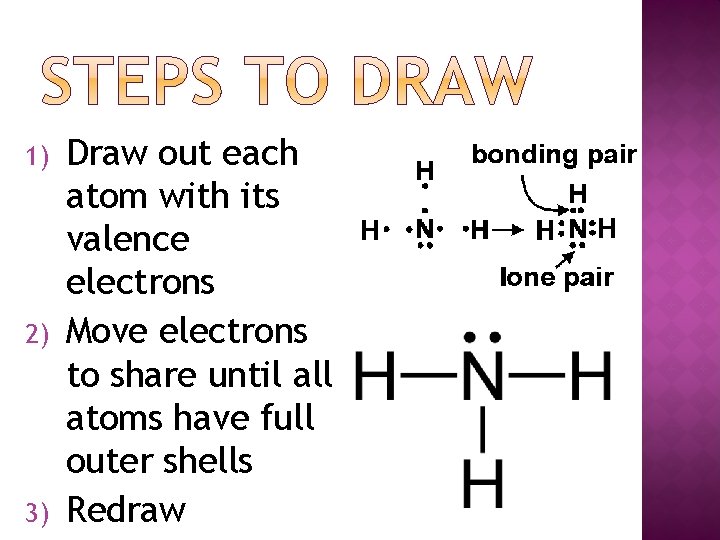
1) 2) 3) Draw out each atom with its valence electrons Move electrons to share until all atoms have full outer shells Redraw
� Put the single atom in the middle of the dot structure � Hydrogen is NEVER in the middle of the dot structure � A dash connecting two atoms represents a pair of shared electrons Ø Sometimes, more than one pair of electrons is shared v v Two pairs = double bond Three pairs = triple bond � Don’t forget to re-draw non-bonding electrons!
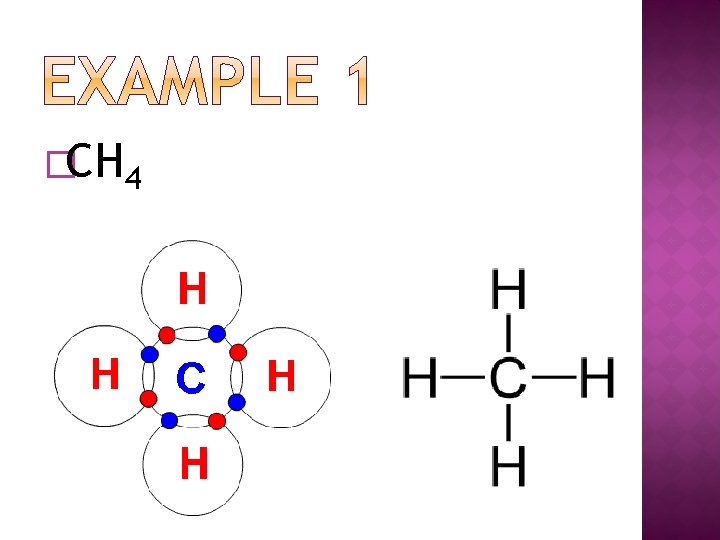
�CH 4
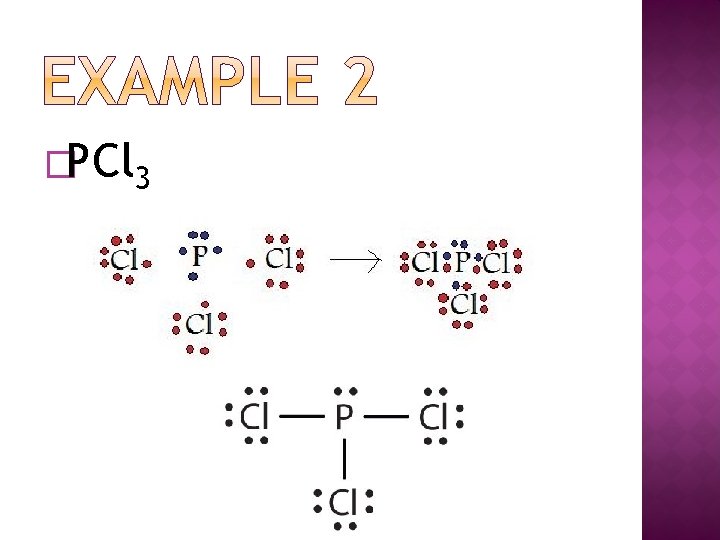
�PCl 3
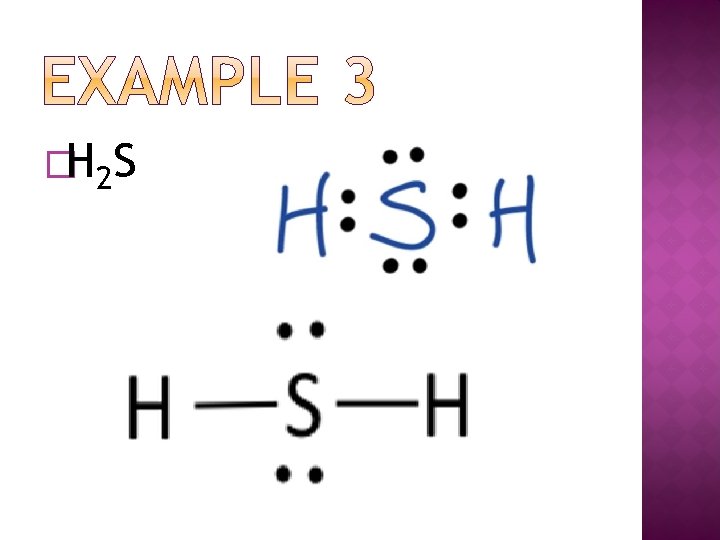
�H 2 S
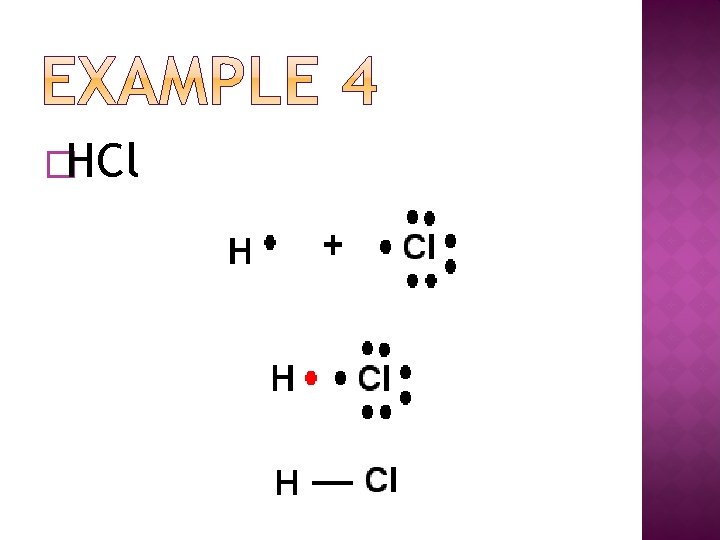
�HCl
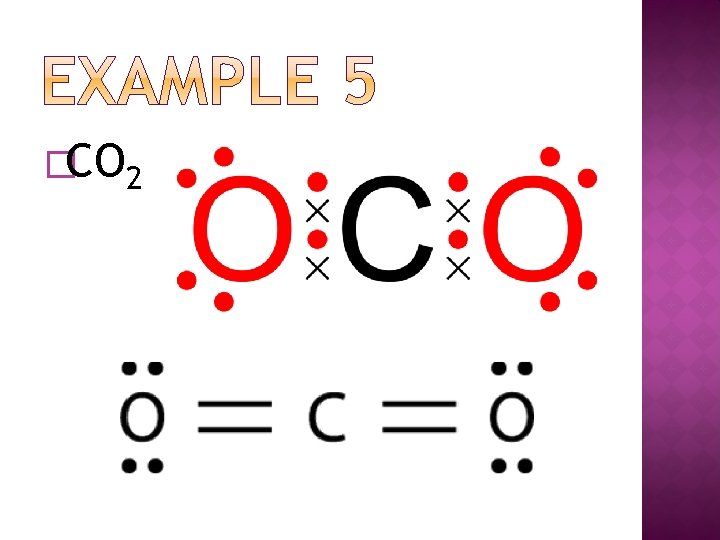
�CO 2
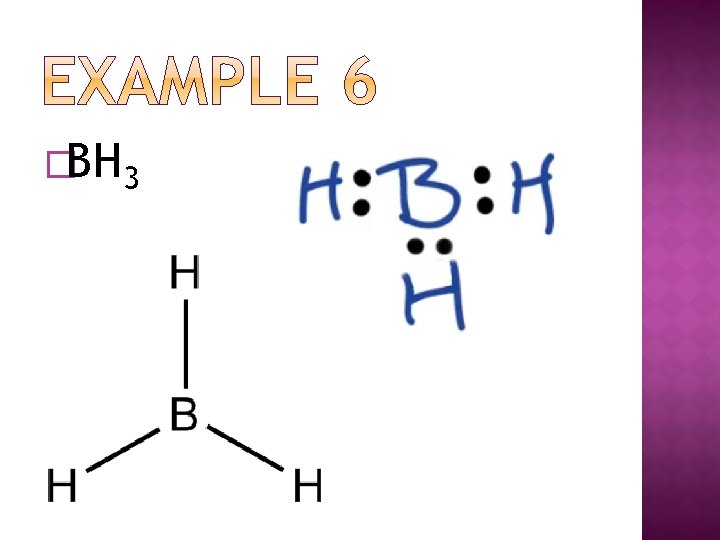
�BH 3
- Slides: 16