What impact does the COVID19 pandemic have on








- Slides: 14

What impact does the COVID-19 pandemic have on ongoing trials: An example in COPD. Dr David Wright, Head of Statistical Innovation, Early Biometrics and Statistical Innovation, Bio. Pharmaceuticals R&D, Astra. Zeneca, Cambridge, UK

Acknowledgements • Thanks to the EFPIA/EFSPI Estimands Implementation and Pharmaceutical Industry COVID-19 Biostatistics Working Groups for help forming some of the views expressed here • Particular thanks to Oliver Keene (GSK) for discussions and comments on the slides and Patrick Darken, Ian Hirsch and Chris Miller (Astra. Zeneca) for comments on the slides • But slides are my own views and don’t necessarily represent the views of any of the above people, Companies or groups! 2

Contents • Note I am not discussing issues with trials set up for investigating treatments to alleviate symptoms/prevent COVID-19 • Assess impact on • • • Research question/ clinical objective Primary estimand Intercurrent events (ICEs) Summary measure Missing Data Estimator and Estimation • All of the above will be illustrated with an example of a fictitious COPD trial 3

What was the original research question/clinical objective • Q 1: Are we interested in how drug A compares to control in the absence of the pandemic? • i. e. how the drug would be used in the future if the pandemic is either no longer an issue (due to vaccination/effective treatments) or because some of the operational issues COVID-19 caused in the trial are no longer an issue now/in the immediate future. • Or are we interested in how drug A compares to control in the presence of the infections due to COVID-19 and/or the operational effects caused by the pandemic • From above important to realise COVID-19 has many different impacts on a study. • Operational effects were extreme due to lockdown measures. These effects are unlikely to apply in general in the future. Therefore it doesn’t make sense to evaluate efficacy using an estimand that includes those measures if interested in Q 1. 4

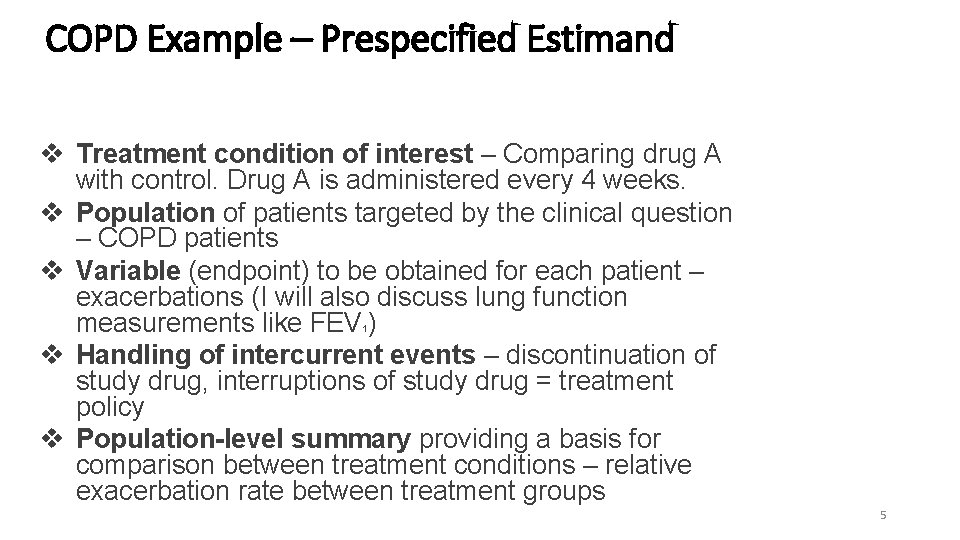
COPD Example – Prespecified Estimand v Treatment condition of interest – Comparing drug A with control. Drug A is administered every 4 weeks. v Population of patients targeted by the clinical question – COPD patients v Variable (endpoint) to be obtained for each patient – exacerbations (I will also discuss lung function measurements like FEV ) v Handling of intercurrent events – discontinuation of study drug, interruptions of study drug = treatment policy v Population-level summary providing a basis for comparison between treatment conditions – relative exacerbation rate between treatment groups 1 5

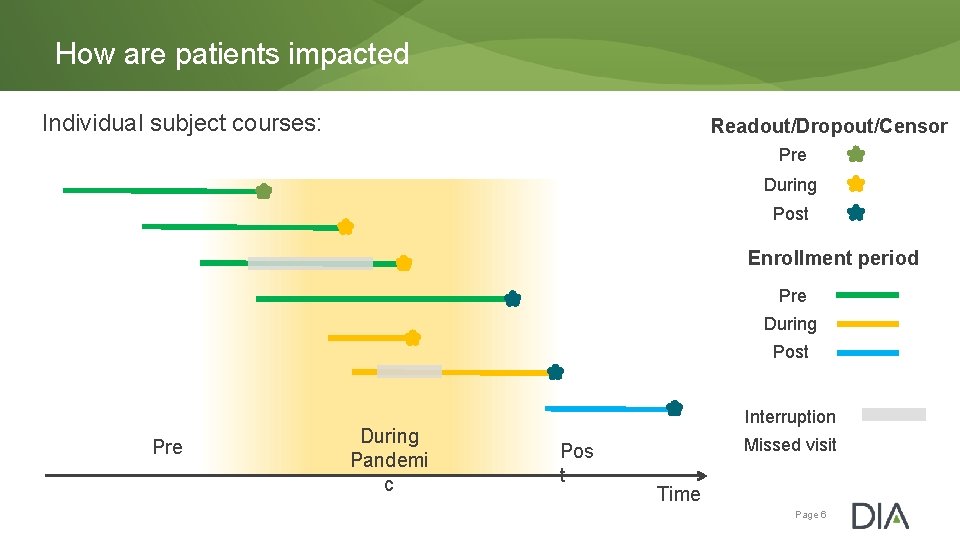
How are patients impacted Individual subject courses: Readout/Dropout/Censor Pre During Post Enrollment period Pre During Post Pre During Pandemi c Interruption Pos t Missed visit Time Page 6

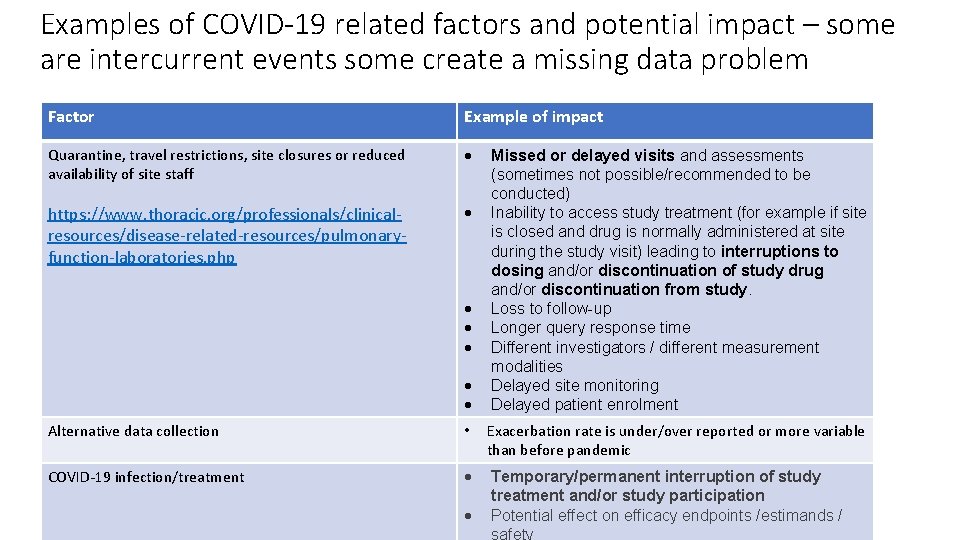
Examples of COVID-19 related factors and potential impact – some are intercurrent events some create a missing data problem Factor Example of impact Quarantine, travel restrictions, site closures or reduced availability of site staff https: //www. thoracic. org/professionals/clinicalresources/disease-related-resources/pulmonaryfunction-laboratories. php Missed or delayed visits and assessments (sometimes not possible/recommended to be conducted) Inability to access study treatment (for example if site is closed and drug is normally administered at site during the study visit) leading to interruptions to dosing and/or discontinuation of study drug and/or discontinuation from study. Loss to follow-up Longer query response time Different investigators / different measurement modalities Delayed site monitoring Delayed patient enrolment Alternative data collection • Exacerbation rate is under/over reported or more variable than before pandemic COVID-19 infection/treatment Temporary/permanent interruption of study treatment and/or study participation Potential effect on efficacy endpoints /estimands / safety

How to answer the question how does drug A compare to placebo in the absence of the pandemic • Option 0: Make no changes to original planned analyses. • Option 1: Only look at data collected before pandemic started (or patients recruited after it is agreed the pandemic is over) • • Could be appropriate if COVID-19 has had hardly any impact on the study. However treatment policy strategy not supported for pandemic related intercurrent events as these events are unlikely to occur in the future. Therefore the treatment effect in these circumstances will be of limited (or no) relevance. Only feasible as an option in a study that had virtually finished before the pandemic started. Most trials will be severely underpowered or severely delayed if taking this option. Option 2: Use data on all patients but handle pandemic related ICEs differently to ICEs not related to the pandemic • For example, dosing interruptions are unusual in clinical trials and dosing interruptions even less likely to occur in the future if the drug is approve and prescribed. Hence using a hypothetical strategy for what would have happened if dosing had not been interrupted due to COVID-19 pandemic (an operational issue) is recommended. What if dosing is interrupted due to patient being infected with COVID-19? I would argue a hypothetical approach is justified here too. 8

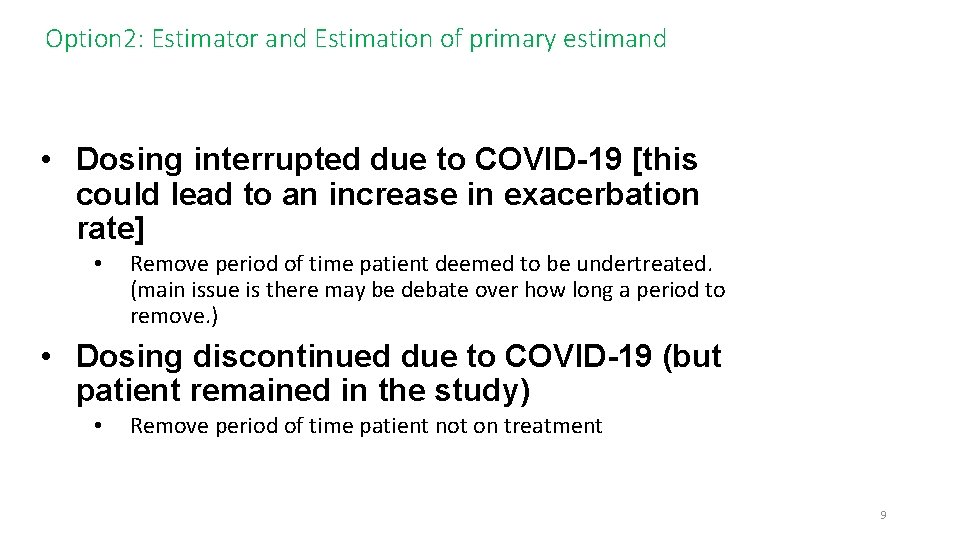
Option 2: Estimator and Estimation of primary estimand • Dosing interrupted due to COVID-19 [this could lead to an increase in exacerbation rate] • Remove period of time patient deemed to be undertreated. (main issue is there may be debate over how long a period to remove. ) • Dosing discontinued due to COVID-19 (but patient remained in the study) • Remove period of time patient not on treatment 9

Impact on exacerbation data due to pandemic • Exacerbations missed due to pandemic? • Population changes • Subjects could become more severely ill due to pandemic adversely impacting on their patient care – observed rate increases • Subjects less likely to present at hospital due to concerns over going to hospital due to COVID-19, less oral steroids could then be prescribed – observed rate decreases 10

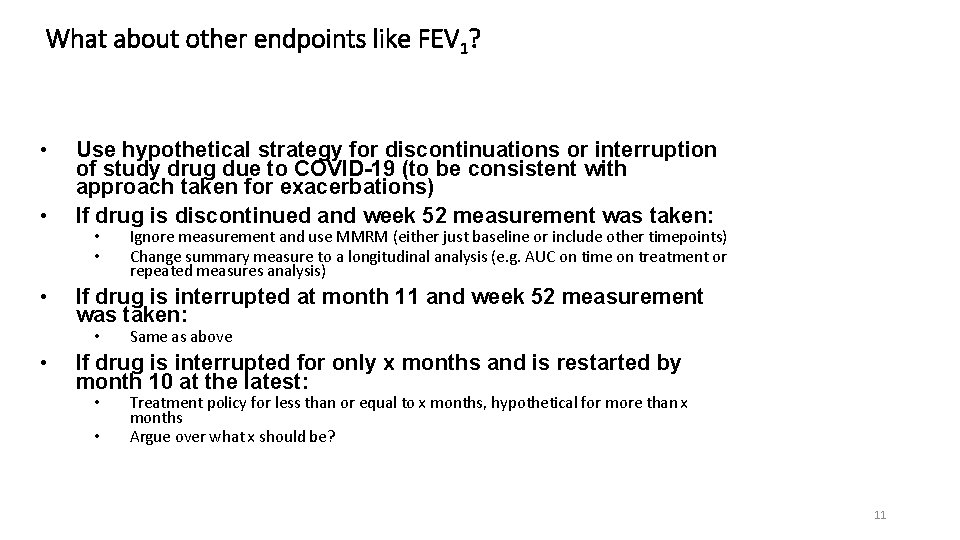
What about other endpoints like FEV 1? • • • Use hypothetical strategy for discontinuations or interruption of study drug due to COVID-19 (to be consistent with approach taken for exacerbations) If drug is discontinued and week 52 measurement was taken: • • If drug is interrupted at month 11 and week 52 measurement was taken: • • Ignore measurement and use MMRM (either just baseline or include other timepoints) Change summary measure to a longitudinal analysis (e. g. AUC on time on treatment or repeated measures analysis) Same as above If drug is interrupted for only x months and is restarted by month 10 at the latest: • • Treatment policy for less than or equal to x months, hypothetical for more than x months Argue over what x should be? 11

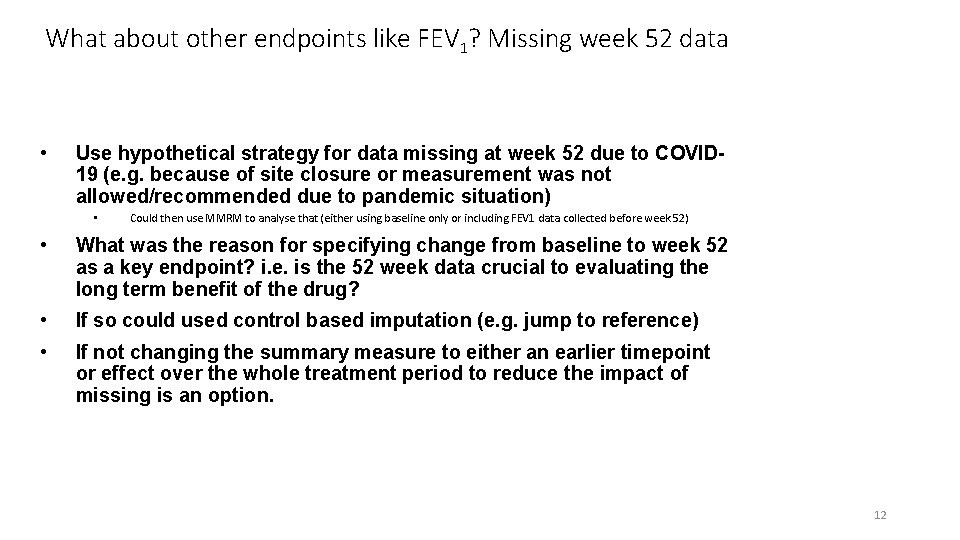
What about other endpoints like FEV 1? Missing week 52 data • Use hypothetical strategy for data missing at week 52 due to COVID 19 (e. g. because of site closure or measurement was not allowed/recommended due to pandemic situation) • Could then use MMRM to analyse that (either using baseline only or including FEV 1 data collected before week 52) • What was the reason for specifying change from baseline to week 52 as a key endpoint? i. e. is the 52 week data crucial to evaluating the long term benefit of the drug? • If so could used control based imputation (e. g. jump to reference) • If not changing the summary measure to either an earlier timepoint or effect over the whole treatment period to reduce the impact of missing is an option. 12

COVID-19 deaths • Be precise: this could be people dying of COVID-19 infection or people dying due to problems in the healthcare system as a result of the pandemic or people dying of the underlying medical condition whilst being infected with COVID-19). • Ascertainment of whether or not death was caused by COVID-19 is problematic • Safety analysis could be complex • High increase in deaths could lead to questions over how death is handled in exacerbation and FEV 1 analysis 13

Conclusion • If primary question of interest is what is the benefit of treatment in the absence of the pandemic using a treatment policy approach for intercurrent events and missing data related to the pandemic is generally not appropriate. • Instead using a consistent hypothetical strategy for pandemic related intercurrent events and missing data should be seriously considered and discussed with regulators • • If used important to understand how this estimand will be estimated and whether that is feasible particularly if faced with a large amount of missing data Other options like changing the summary measure to one that can be more reliably be estimated should also be considered. 14
 Http://apps.tujuhbukit.com/covid19/
Http://apps.tujuhbukit.com/covid19/ Do if you covid19
Do if you covid19 Covid19 athome rapid what know
Covid19 athome rapid what know What do if test positive covid19
What do if test positive covid19 Vaksin covid19
Vaksin covid19 Covid 19 pandemic summary
Covid 19 pandemic summary Who pandemic phases
Who pandemic phases Pandemic tabletop exercise template
Pandemic tabletop exercise template Mathalicious pandemic answer key
Mathalicious pandemic answer key Global pandemic preparedness
Global pandemic preparedness What causes wind to blow brainpop
What causes wind to blow brainpop What impact does a tornado have on an ecosystem?
What impact does a tornado have on an ecosystem? What shape has 8 faces and 12 vertices
What shape has 8 faces and 12 vertices Does congress have the power to stop mail on saturdays
Does congress have the power to stop mail on saturdays Role of gaa in irish life
Role of gaa in irish life