WHAT HOSPITALS SHOULD KNOW ABOUT PHARMACEUTICAL WASTE COMPLIANCE

WHAT HOSPITALS SHOULD KNOW ABOUT PHARMACEUTICAL WASTE COMPLIANCE Presented at: Hospitals for a Healthy Environment in Rhode Island’s Second Annual Conference John La. Carrubba April 2, 2012

PHARMACEUTICAL WASTE MANAGEMENT Regulations & References The information provided in this presentation is based on the referenced Code of Federal Regulations. This data is presented only as a reference. For complete requirements or legal counsel on hazardous waste regulations and interpretations, generators should consult their legal department, the applicable Code of Federal Regulations and applicable State regulatory agencies. Disclosure Declaration As a Stericycle employee, I have a vested interest in and affiliation with a corporate organization offering financial support or grant monies for this continuing education activity and a business interest in pharmaceutical waste management services.

PHARMACEUTICAL WASTE MANAGEMENT A Growing Concern Media Coverage • 9/15/08 USA Today/AP report - “Hospitals dumping drugs into water” • 5/24/10 Modern Healthcare - “Drugged” • 3/15/12 Modern Medicine – “Hospitals at risk for hazardous pharmaceutical waste violations” EPA Regulatory Activity • Notice of Violations and warnings • Increasing regulatory scrutiny country wide • Fines in excess of $450, 000 The Joint Commission (TJC) • • Medication Management Environment of Care Emergency Management Leadership standards

PHARMACEUTICAL WASTE THE ENFORCERSMANAGEMENT EPA Rx Waste Determination Generator Status EPA ID Number Satellite Accumulation Area Central Accumulation Area Documented Training DOT Classification, description, and packaging (173. 22) Proper marking and labeling (172. 300) Segregation into proper streams (173. 21) Training (172. 202 & 172. 204) TJC Hospital has written plan for managing hazardous waste Maintains written inventory of hazardous materials The hospital complies with Publicly Owned Treatment Works (POTW)law and regulation STATE REGULATORY AGENCIES (RIDEM)

PHARMACEUTICAL WASTE MANAGEMENT Common EPA Inspection Issues • Hazardous waste determinations not done or incorrect • Labeling of hazardous waste not done or incorrect • Disposing HW down the drain, in red bag, in solid waste • No or inadequate HW manifests • Improper disposal of chemotherapy drugs • Inadequate training for employees in HW management • Not conducting proper weekly inspections of HW storage • Lack of emergency contingency plan • Improper management of expired pharmaceuticals “Identification and Management of Regulated Hazardous Waste” – EPA Region 2

TJC Accreditation PHARMACEUTICAL WASTE MANAGEMENT MM. 01. 03 - MEDICATION MANAGEMENT • The hospital safely manages high-alert and hazardous medications • The hospital has a process in place that addresses how outside resources, if any, are used for the destruction of pharmaceuticals. EC. 02. 01 - ENVIRONMENT OF CARE • The hospital manages its hazardous materials wastes risks. LD. 04. 01 - LEADERSHIP • The hospital complies with law and regulation. EM. 02. 05 – EMERGENCY MANAGEMENT • The organization prepares for how it will manage hazardous materials and waste. The Joint Commission under their elements of performance require proper management of hazardous materials including pharmaceutical waste (Standard EC. 02. 01, EP 8)

PHARMACEUTICAL WASTE MANAGEMENT What are they regulating? EPA (RCRA) Two (2) Categories of RCRA Hazardous Waste: Listed and Characteristic Listed Wastes • P – Listed = Acutely Hazardous Coumadin, Nicotine, Physostigmine, Arsenic Trioxide Epinephrine*, Nitroglycerin* *Requires State adoption of US EPA interpretations • U-Listed = Toxic (chemotherapy) P-List U-List

PHARMACEUTICAL WASTE MANAGEMENT What are they regulating? EPA (RCRA) Characteristic Hazardous Ignitable, Corrosive, Reactive, Toxic • • Lantus Humalog Humulin N&R Novolog • Flovent, Albuterol, Combivent, Cetacaine, Dermoplast Spray, Advair HFA Incompatible Hazardous Waste RCRA Incompatible drugs are those that CANNOT be placed in the same container without danger of a chemical reaction. (e. g. Corrosives and Oxidizers) Debrox Unused Silver Nitrate
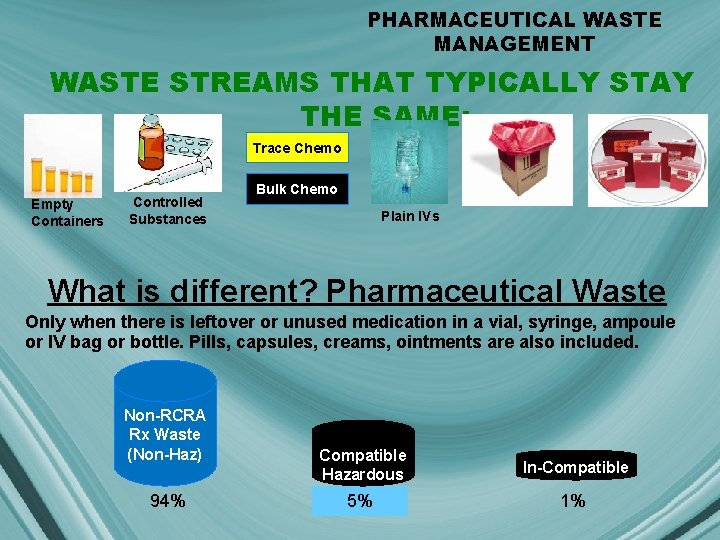
PHARMACEUTICAL WASTE MANAGEMENT WASTE STREAMS THAT TYPICALLY STAY THE SAME: Trace Chemo Empty Containers Controlled Substances Bulk Chemo Plain IVs What is different? Pharmaceutical Waste Only when there is leftover or unused medication in a vial, syringe, ampoule or IV bag or bottle. Pills, capsules, creams, ointments are also included. Non-RCRA Rx Waste (Non-Haz) 94% Compatible Hazardous In-Compatible 5% 1%

PHARMACEUTICAL WASTE MANAGEMENT What Happens To Hospital Rx Inventory? What is Pharmaceutical waste? – Dispensed to patients – Return to manufacturer for credit – Rx Waste Ø No longer used for its intended purpose ØTo be discarded • Partial vials • Partial syringes • Full or partial pre-instilled IV’s • Discontinued/un-administered meds • Hospital repacks • Patient prescriptions • Pre-filled syringes • Samples

PHARMACEUTICAL WASTE MANAGEMENT EPA RCRA Trace, Empty and Bulk Trace • Used to describe RCRA empty containers RCRA Empty - Both conditions must be met: • All contents removed via commonly employed practices (pouring, pumping, aspirating) • Less than 3% of original weight by capacity if the container is less than or equal to 119 gal Bulk • More than “trace”

PHARMACEUTICAL WASTE MANAGEMENT Federal EPA Waste Generator Status CESQG Conditionally Exempt Small Quantity Generator < or = 100 kg/mo (220 lbs)of non-acute hazardous waste < 1 kg/month (2. 2 lbs) of acute hazardous waste (P-Listed)* Small Quantity Generator * Between 100 kg (220 lbs) and 1000 kg/mo (2200 lbs)of non-acute hazardous waste < 1 kg/month (2. 2 lbs) of acute hazardous waste (P-Listed) Large Quantity Generator > = 1000 kg/mo (2200 lbs) of non-acute hazardous waste > = 1 kg/month (2. 2 lbs) acute hazardous waste (P-Listed) * SQG status must be verified & documented monthly

TRAINING PHARMACEUTICAL WASTE MANAGEMENT RCRA Training • Employees involved with or occupationally exposed to hazardous waste • Completed within 6 months • Annual retraining • Record retention requirement Hazard Communication Training • Employees involved with or occupationally exposed to hazardous chemicals must be trained in accordance with 29 CFR 1910. 200 • Completed at time of initial assignment to job DOT Training • Employees involved with or occupationally exposed to hazardous materials must be trained in accordance with 49 CFR Subpart H 265 (172. 702 & 172. 704) • Completed within 90 days • Retraining every three years • Record retention requirement

Staff Education PHARMACEUTICAL WASTE MANAGEMENT Implementation Training EVS Pharmacy Unit Specific Nursing/Clinicians OR & ED Training Topics Regulatory requirements Waste segregation Aftercare On-site follow-up Monthly & quarterly schedule Refresher Training RX Waste containers Transportation & Disposal

PHARMACEUTICAL WASTE MANAGEMENT GETTING STARTED 1. Understand the need for a pharmaceutical waste program based on regulatory involvement and environmental concerns. 2. Evaluate current handling practices of pharmaceutical waste in comparison to federal and state regulations. 3. Identify a group of leaders in your facility that have a passion for the environment, will champion multi-departmental cooperation and administration support.

PHARMACEUTICAL WASTE MANAGEMENT A Team Effort Departments with champions that advocate for compliant and environmentally responsible pharmaceutical waste disposal: Pharmacy Nursing Education Quality/Accreditation Safety Environmental Services Risk Management Infection Control Facilities Management Public Relations

PHARMACEUTICAL WASTE MANAGEMENT PROGRAM IMPLEMENTATION COMMUNICATE, COMMUNICATE !!! Internal communication – Talk it up • Intranet ● Coming Soon Notices • Web-site ● Management Meetings • Newsletter ● Unit Huddles Communicate to staff BEFORE implementation • Program announcements – Who, What & Why • Training dates • Program start date External communication UPON implementation • Press releases - "Green Initiative” • Assuring regulatory compliance • Environmental stewardship – “The right thing to do” • Employee & community safety

PHARMACEUTICAL WASTE MANAGEMENT Questions
- Slides: 18