What do you remember about how elements form
What do you remember about how elements form compounds? Come write answers on the board
Main Points - Normally Formed from a transfer of electrons - Positive or Negative ions want to become neutral - Some ions are more readily available for reactions than other - Some compounds are electrolytes (ionic) - Some compounds are nonelectrolytes (molecular)

Refresher www. youtube. com/watch? v=LRVW 0 tg. SLRI
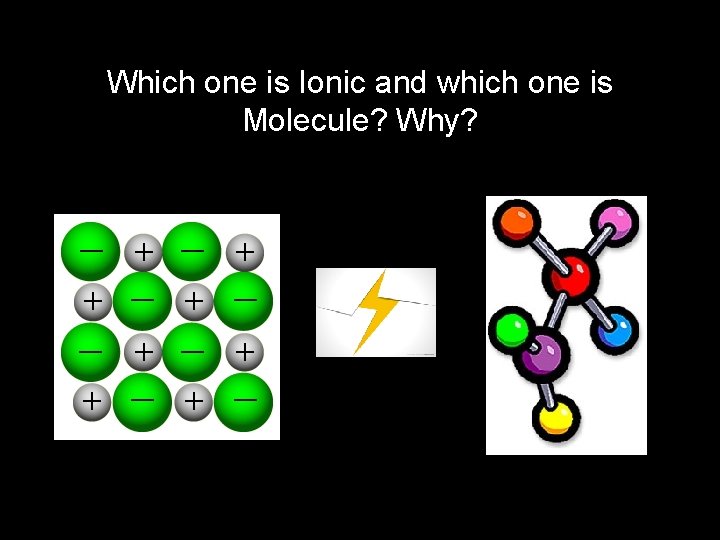
Which one is Ionic and which one is Molecule? Why?
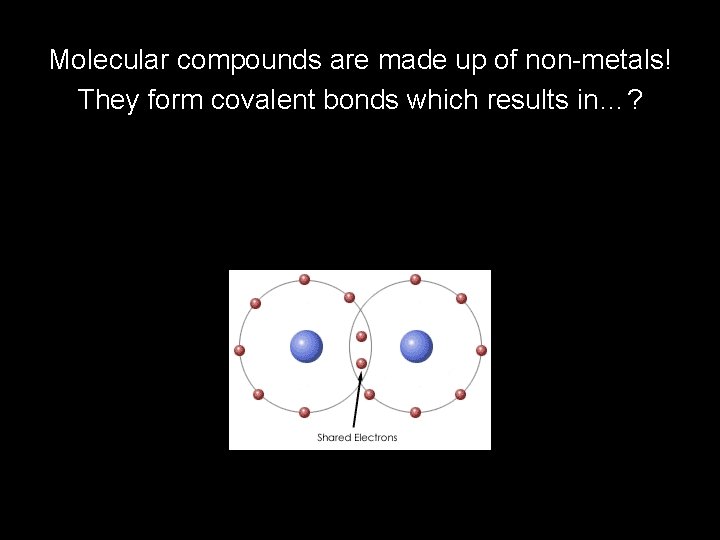
Molecular compounds are made up of non-metals! They form covalent bonds which results in…?
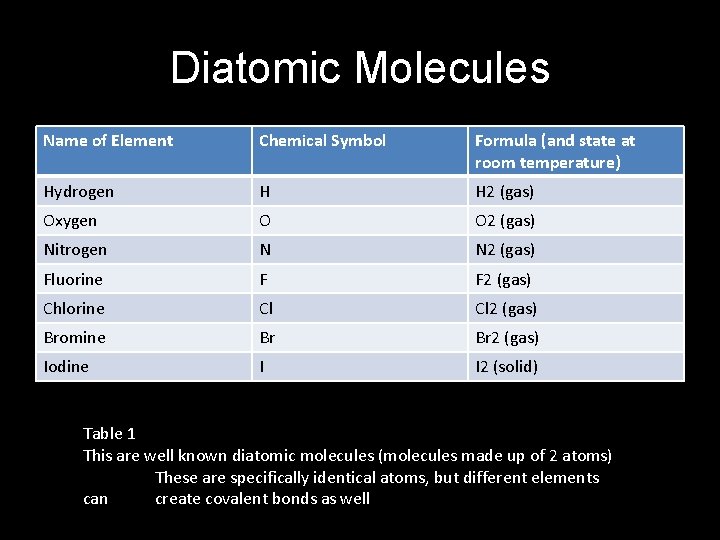
Diatomic Molecules Name of Element Chemical Symbol Formula (and state at room temperature) Hydrogen H H 2 (gas) Oxygen O O 2 (gas) Nitrogen N N 2 (gas) Fluorine F F 2 (gas) Chlorine Cl Cl 2 (gas) Bromine Br Br 2 (gas) Iodine I I 2 (solid) Table 1 This are well known diatomic molecules (molecules made up of 2 atoms) These are specifically identical atoms, but different elements can create covalent bonds as well

Writing formulas Let’s go through the rules on the board (pg. 203) Rule 1: Rule 2: Rule 3: Rule 4:
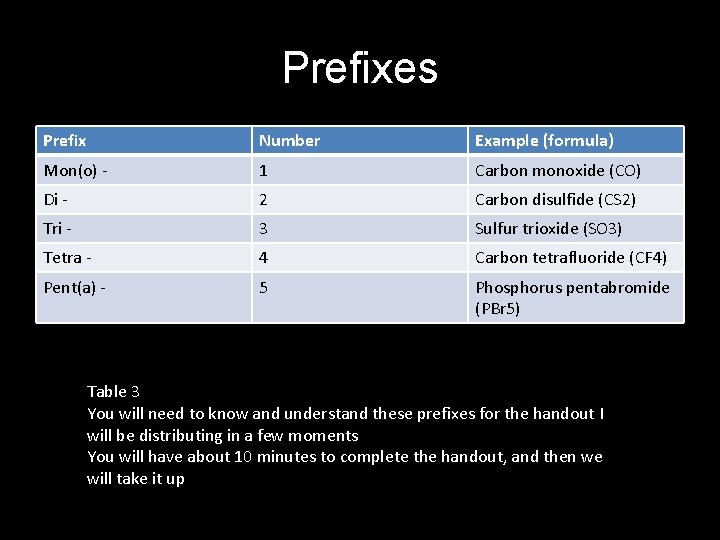
Prefixes Prefix Number Example (formula) Mon(o) - 1 Carbon monoxide (CO) Di - 2 Carbon disulfide (CS 2) Tri - 3 Sulfur trioxide (SO 3) Tetra - 4 Carbon tetrafluoride (CF 4) Pent(a) - 5 Phosphorus pentabromide (PBr 5) Table 3 You will need to know and understand these prefixes for the handout I will be distributing in a few moments You will have about 10 minutes to complete the handout, and then we will take it up

In Class Work: To Be Handed In By the End of Class Pg. 204 Questions 1 -9
- Slides: 9