What do these have in common Learning Outcomes




















- Slides: 23

What do these have in common?

Learning Outcomes ALL will be able to state what is meant by an alloy (level 4) MOST will be able to describe uses of some alloys (level 5) SOME will be able to explain why an alloy can be stronger than a pure metal (level 6)

Literacy in Science • Alloy • Properties • Elements • Mixture

What is an alloy? (LO 1) An alloy is a mixture of at least two elements, where at least one of these is a metal ‘rose gold’copper and gold

What is an alloy? (LO 1) New, more useful materials can be created in this way Alloys have a mixture of the properties of the original elements ‘Birmabright’aluminium and magnesium

Useful alloys (LO 2) Brass is formed from copper and zinc and is much stronger than both

Useful alloys (LO 2) Pewter is formed from tin and lead

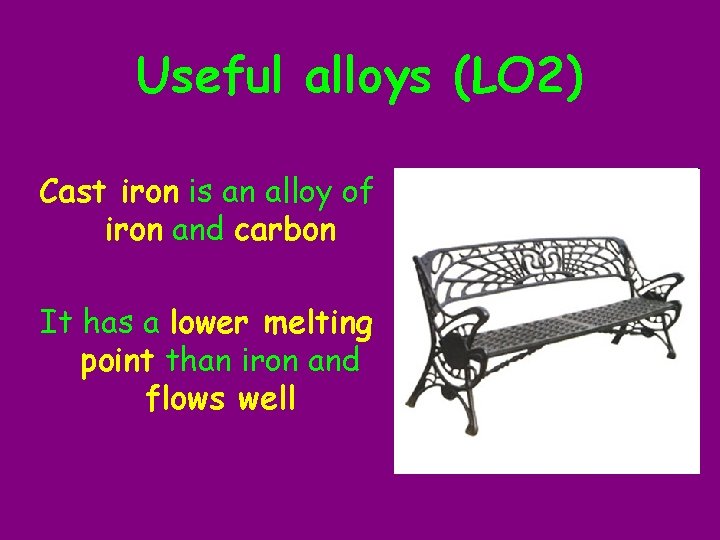
Useful alloys (LO 2) Cast iron is an alloy of iron and carbon It has a lower melting point than iron and flows well

Useful alloys (LO 2) Stainless steel is an alloy of iron, chromium and nickel

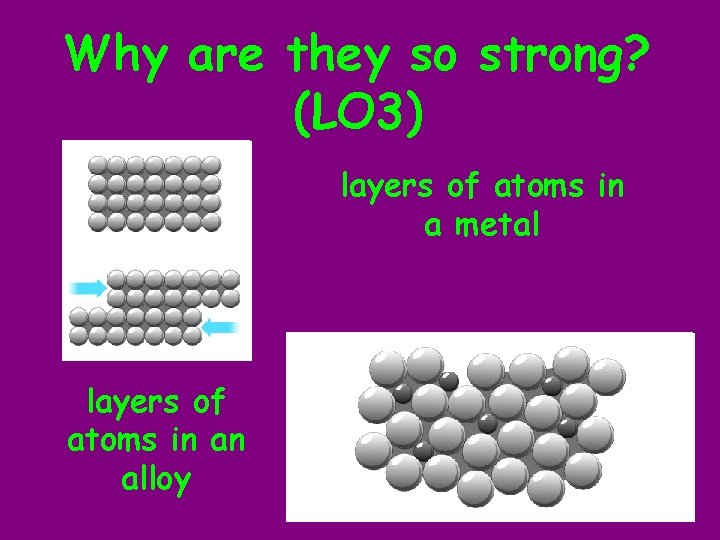
Why are they so strong? (LO 3) layers of atoms in a metal layers of atoms in an alloy

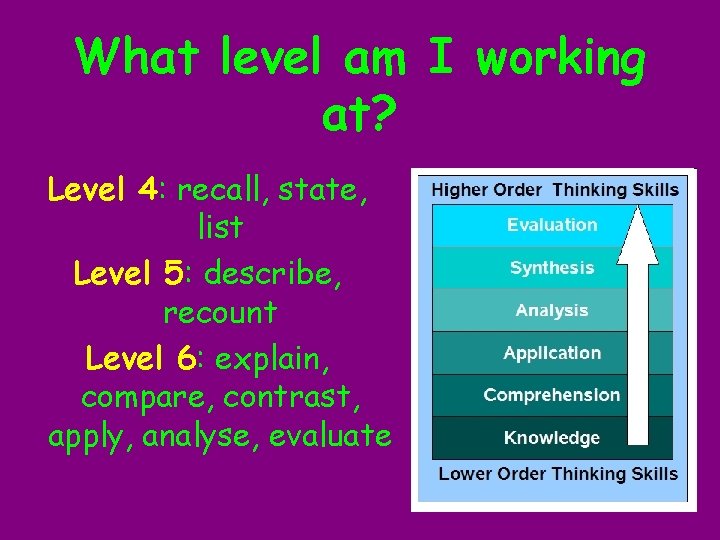
What level am I working at? Level 4: recall, state, list Level 5: describe, recount Level 6: explain, compare, contrast, apply, analyse, evaluate

Peer Assessment Fill the Gap alloy; properties; stronger; rust; mixed; stainless; pure; layers; cannot; more LEVEL 4

Peer Assessment ‘An alloy is a mixture of at least two elements, where at least one of these is a metal’ LEVEL 4

Peer Assessment 2. Example: • Birmabright • Made from aluminium and magnesium • Used for the body of Land Rovers LEVEL 4 -5

Peer Assessment 3. Example: ‘Birmabright is more suitable for its purpose than either of its constituent elements, magnesium and aluminium, because it is stronger and lighter than either. ’ LEVEL 5

Peer Assessment 4 a. Malleable = capable of being shaped or formed (e. g. by hammering) LEVEL 4

Peer Assessment 4 b. The larger atoms/ particles in the alloy prevent the smaller ones from forming layers as well, which means they can’t slip as easily when a force is applied. ’ LEVEL 6

Quiz!

Question One 1. Is an alloy: a. A mixture of two metals b. A mixture of two or more elements, one of which is a metal c. A metal element d. A compound of two non- metals

Question Two 2. Which of these is an alloy? a. Wood b. Aluminium c. Plastic d. Cast iron

Question Three 3. Do alloys have? a. The same properties as one of the original elements b. A mixture of properties of the original elements c. Completely different properties to the original elements

Question Four 4. The alloy brass is a mixture of which two metals? a. Copper and zinc b. Copper and aluminium c. Zinc and iron d. Oxygen and sulphur

Question Five 5. Which of these properties makes cast iron useful for creating intricate shapes? a. Rigid b. Can be recycled c. Nice colour d. Flows well