What do atoms look like What do we




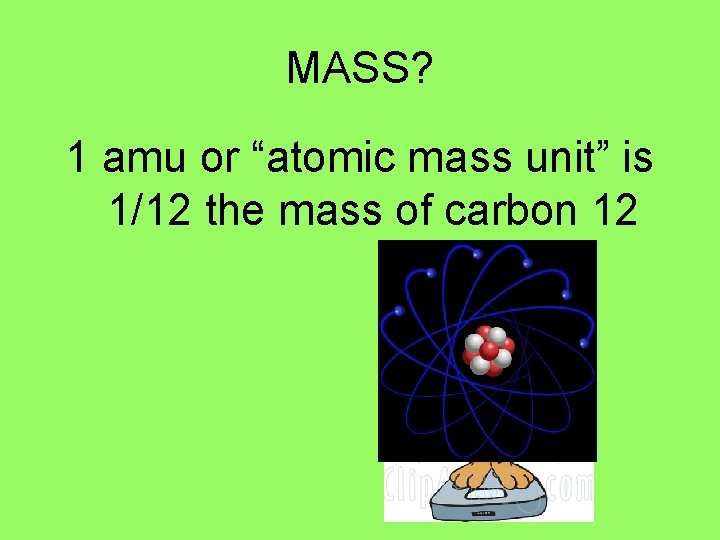






- Slides: 22

What do atoms look like?

What do we know about atoms? *All elements are composed of atoms *The atoms of the same element are the same (and different from the atoms of any other element) *Atoms of different elements can mix together or can chemically combine in a whole number ratio to form compounds * Chemical reactions occur when atoms are joined, separated or rearranged. BUT you can’t turn one element into another by chemical reaction.

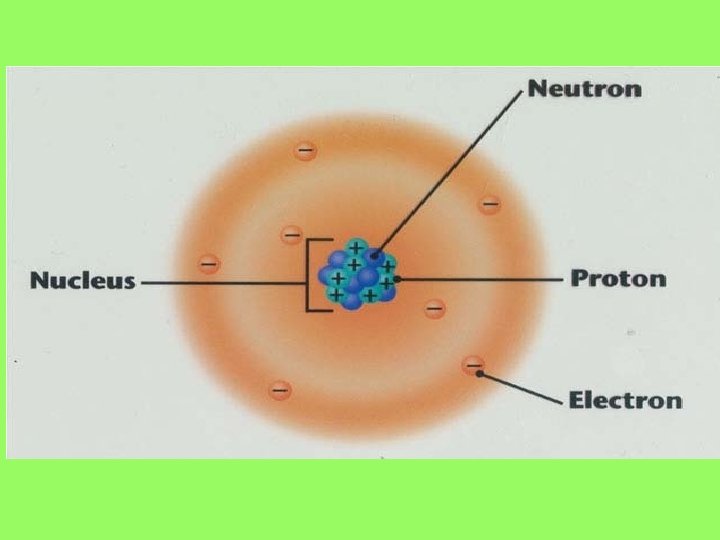
What are atoms made of? • The three basic components of an atom are the – Proton – Neutron – Electron

Where are the subatomic particles found? • Proton • Neutron • electron



• • • Found in the nucleus Has a +1 charge Mass of ~1 amu 10 -15 m in size p+

• • • Found in the nucleus Electrically neutral Mass of ~1 amu 10 -15 m in size n

• Found outside the nucleus in the “electron cloud” • Has a -1 charge • Mass of ~0 • 10 -18 m in size • e-
MASS? 1 amu or “atomic mass unit” is 1/12 the mass of carbon 12

SIZE? • The empty atom: If we imagine the atom’s nucleus to be the size of a bean, the atom itself will become the size of a stadium, and the electrons will be like tiny fleas whizzing frantically somewhere around the stands.

What makes the atoms of one element different from the atoms of another? • The number of protons, neutron, and electrons differs from one element to the next. • The number of determines the element. • The number of protons is equal to the on the periodic table

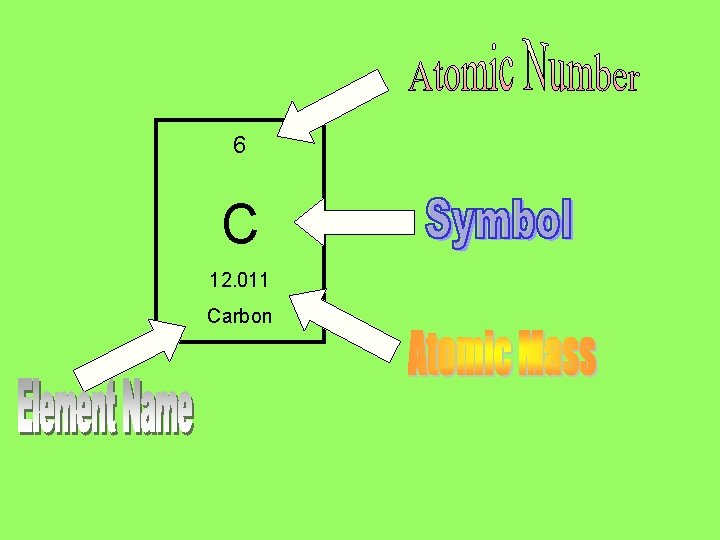
6 C 12. 011 Carbon

Atomic Number = # of Protons • Every Hydrogen atom has ____ protons • Every Magnesium atom has ____ protons • Every Arsenic atom has ____ protons

Atoms are electrically neutral so… # of protons must = # of electrons How many electrons in an atom of Hydrogen? Magnesium? Arsenic?

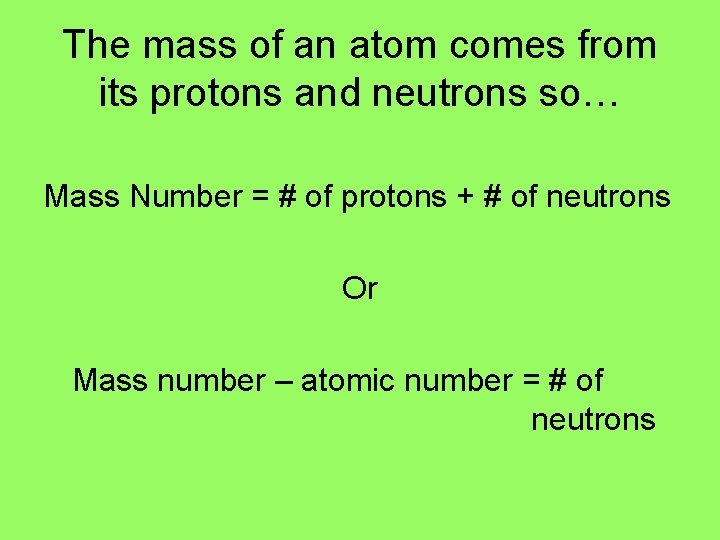
The mass of an atom comes from its protons and neutrons so… Mass Number = # of protons + # of neutrons Or Mass number – atomic number = # of neutrons

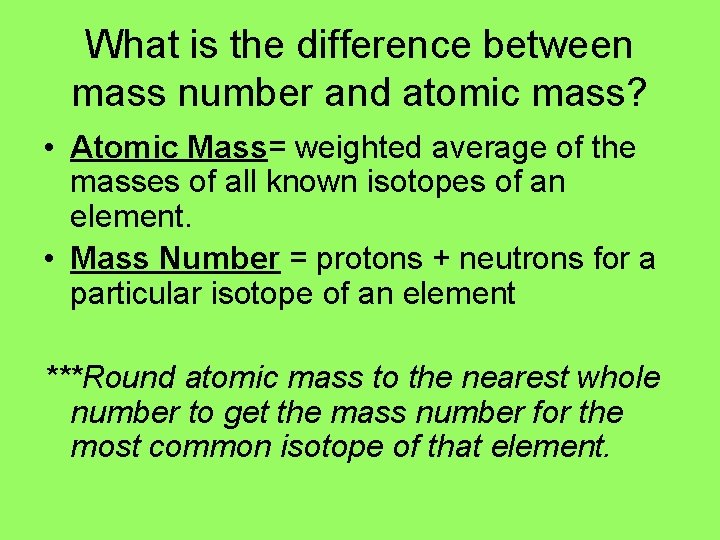
What is the difference between mass number and atomic mass? • Atomic Mass= weighted average of the masses of all known isotopes of an element. • Mass Number = protons + neutrons for a particular isotope of an element ***Round atomic mass to the nearest whole number to get the mass number for the most common isotope of that element.

How many neutrons in an atom of Hydrogen? Magnesium? Arsenic? Round the atomic mass to the nearest whole number

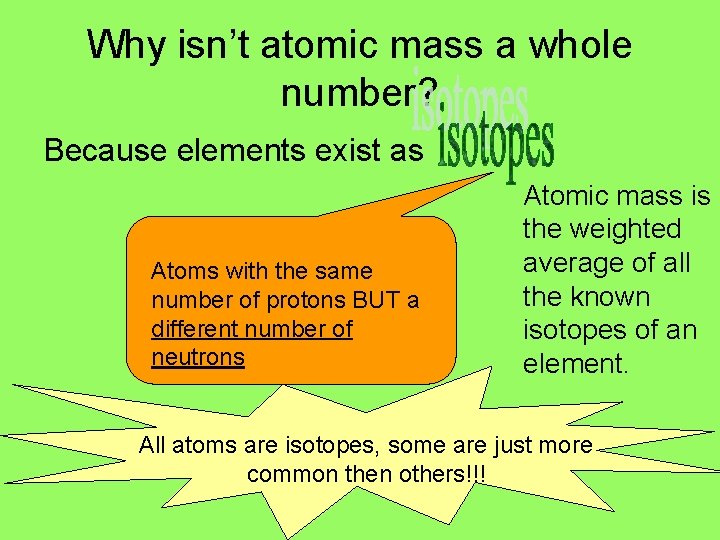
Why isn’t atomic mass a whole number? Because elements exist as Atoms with the same number of protons BUT a different number of neutrons Atomic mass is the weighted average of all the known isotopes of an element. All atoms are isotopes, some are just more common then others!!!

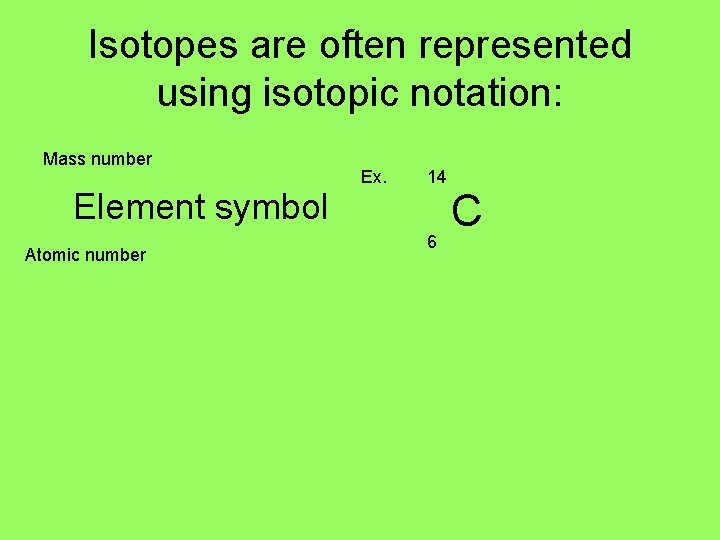
Isotopes are often represented using isotopic notation: Mass number Element symbol Atomic number Ex. 14 6 C

• Many times the atomic number will be left off (since that always remains the same) ex. Carbon-14 or 14 C Did you know that carbon -14 is used to date fossils!

What about IONS? • Ions are electrically charged atoms Positive = cation = more protons then electrons Negative = anion = more electrons then protons Represented as K+1