What distinguishes living organisms 1 Structurally complicated and





- Slides: 25

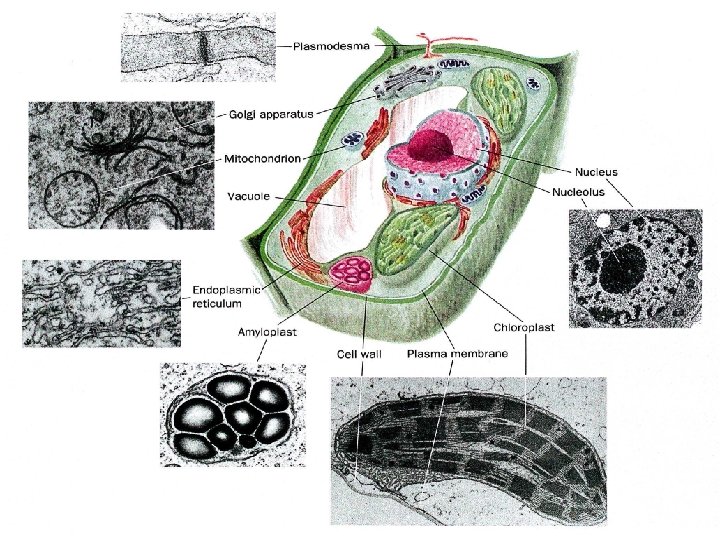
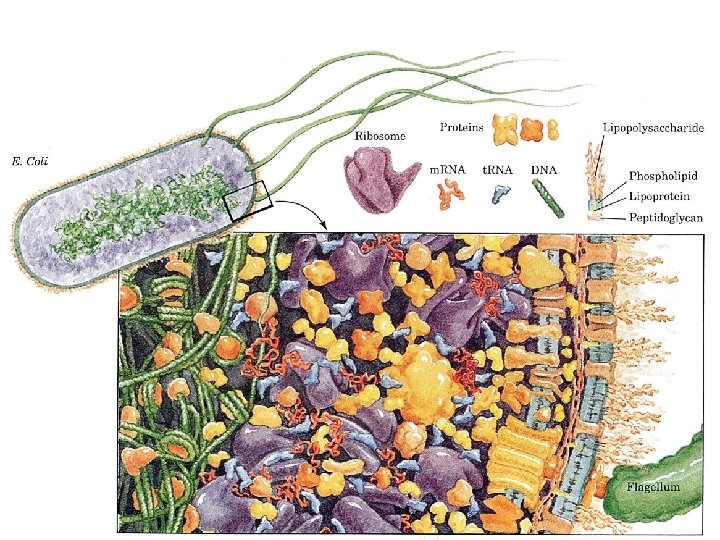
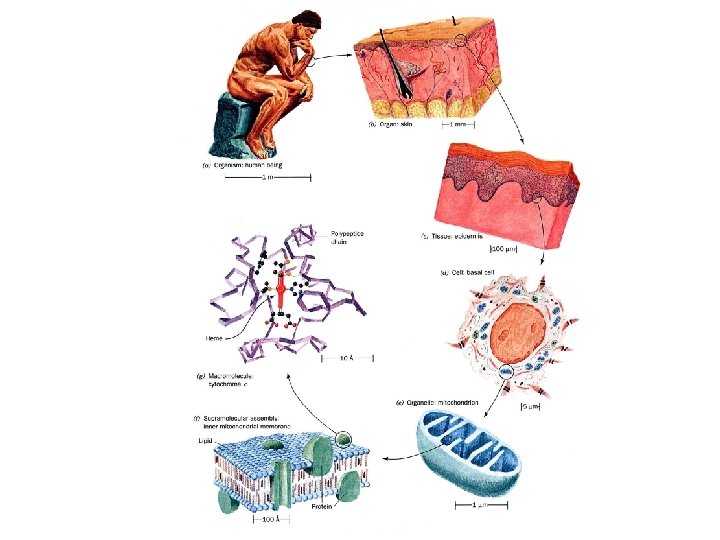
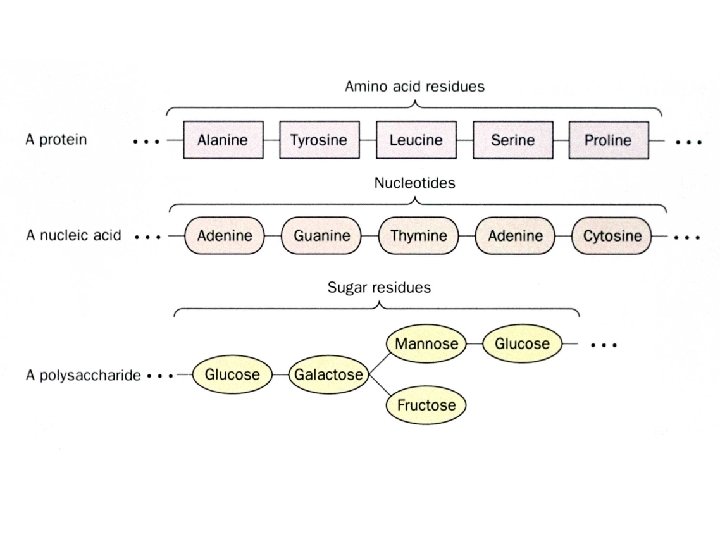
What distinguishes living organisms? 1. Structurally complicated and highly organized a. intricate internal structures b. many kinds of complicated molecules proteins, DNA, RNA, starches, and lipids etc. (inanimate objects sand clay are mixtures of simple compounds) 2) Living organisms: a. extract b. transform c. store d. use ENERGY 1


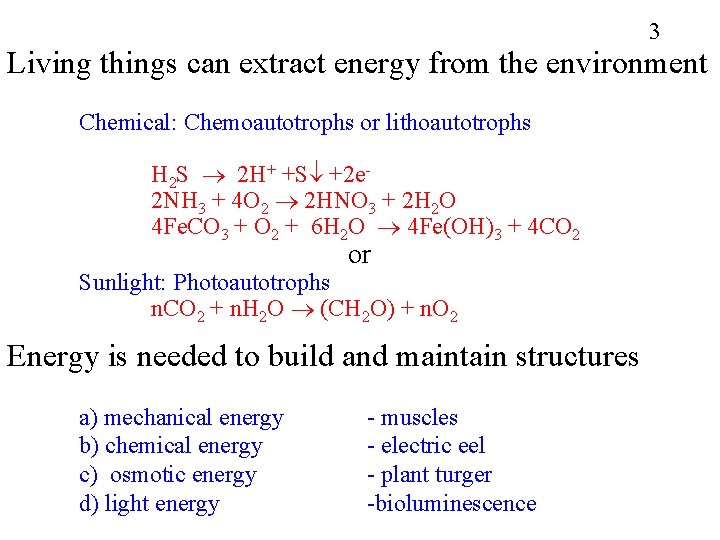
3 Living things can extract energy from the environment Chemical: Chemoautotrophs or lithoautotrophs H 2 S 2 H+ +S +2 e 2 NH 3 + 4 O 2 2 HNO 3 + 2 H 2 O 4 Fe. CO 3 + O 2 + 6 H 2 O 4 Fe(OH)3 + 4 CO 2 or Sunlight: Photoautotrophs n. CO 2 + n. H 2 O (CH 2 O) + n. O 2 Energy is needed to build and maintain structures a) mechanical energy b) chemical energy c) osmotic energy d) light energy - muscles - electric eel - plant turger -bioluminescence

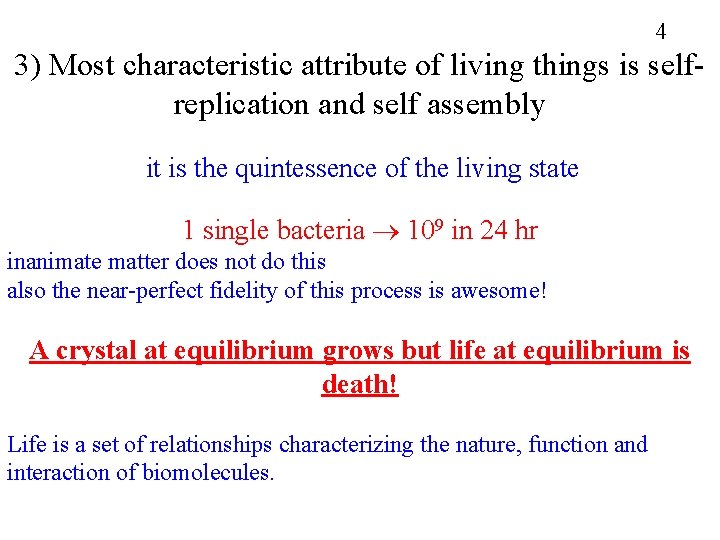
4 3) Most characteristic attribute of living things is selfreplication and self assembly it is the quintessence of the living state 1 single bacteria 109 in 24 hr inanimate matter does not do this also the near-perfect fidelity of this process is awesome! A crystal at equilibrium grows but life at equilibrium is death! Life is a set of relationships characterizing the nature, function and interaction of biomolecules.

5 Philosophers thought life contained a “vital force” or vitalism but this has been rejected by modern science. Important insights and practical applications in medicine, agriculture, nutrition and industry have come from Biochemistry but ultimately biochemistry is still concerned with the WONDER OF LIFE

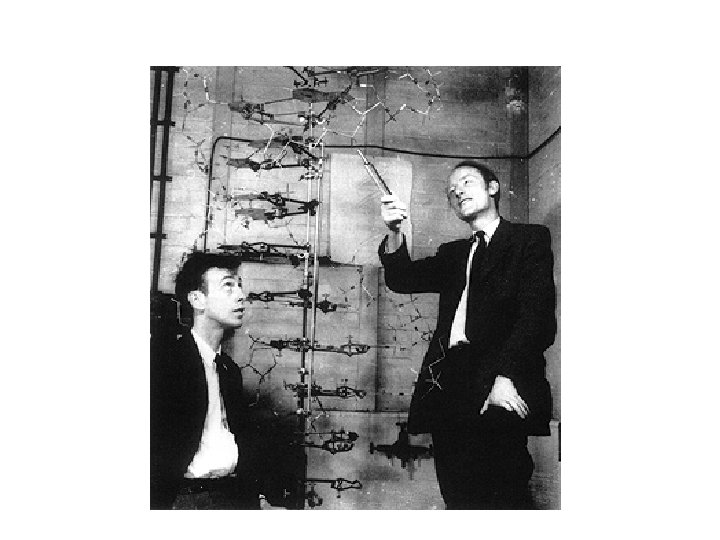
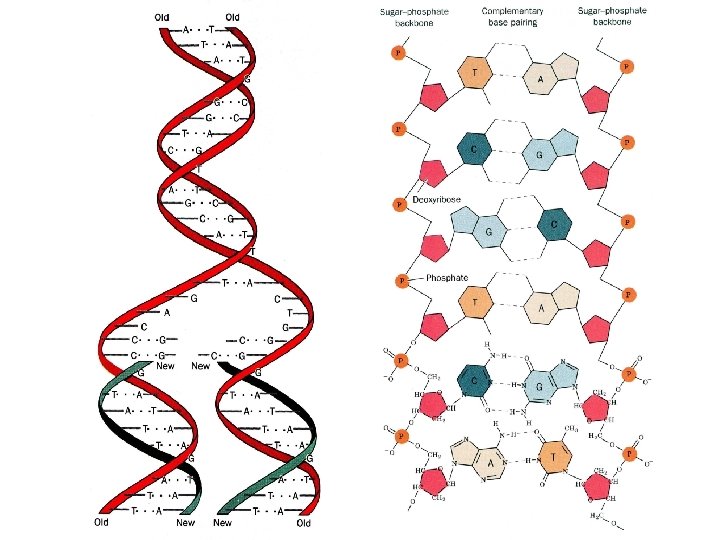
A Brief History of Biochemistry 6 Early 19 th Century World made of either "living matter" (organic) or "non-living matter" (inorganic). (Vitalism) 1828 Friedrich Wohler accomplished the synthesis of Urea from inorganic matter. 1897 Edvard and Hans Buchner showed dead cell extracts can perform reactions of living cells. The molecules responsible for performing these reactions are called enzymes Late 1800's Emil Fischer suggested key/lock picture. Substrate º Key, Enzyme º Lock Early 1900's Field of biochemistry emerges Structure and function of enzymes Elucidating enzymatic pathways 1944 Genes composed of DNA 1953 Watson and Crick determine the structure of DNA Biological function linked to the information in genes

Double helix slide

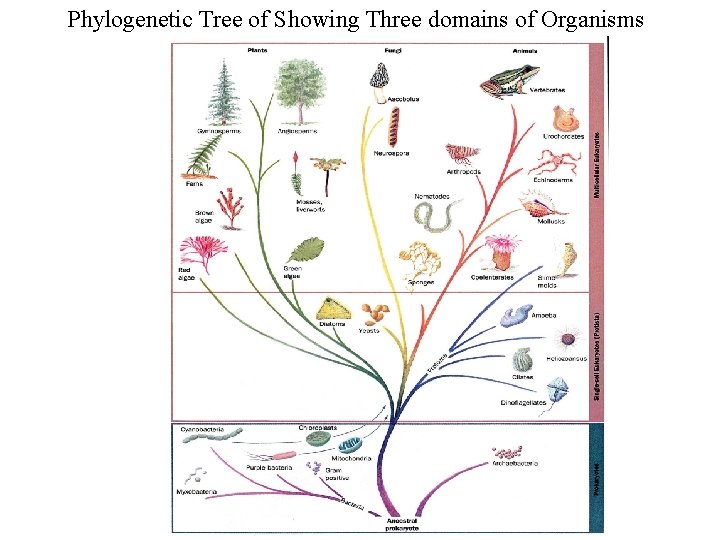
Phylogenetic Tree of Showing Three domains of Organisms

How did organisms evolve? • Blind watchmaker principle, small mutations arise at random. 1. Evolution is not directed 2. Evolution requires in-built sloppiness 3. Evolution is constrained by the past 4. Evolution is ongoing

10 CAN you name a few of the recent discoveries? Range of Life- Hot springs -subduction zones -artic tundra- Antarctic dry fields - from animal intestines to college dormitories. These are all equal to specific biochemical adaptations. Is Life Unique to Earth?





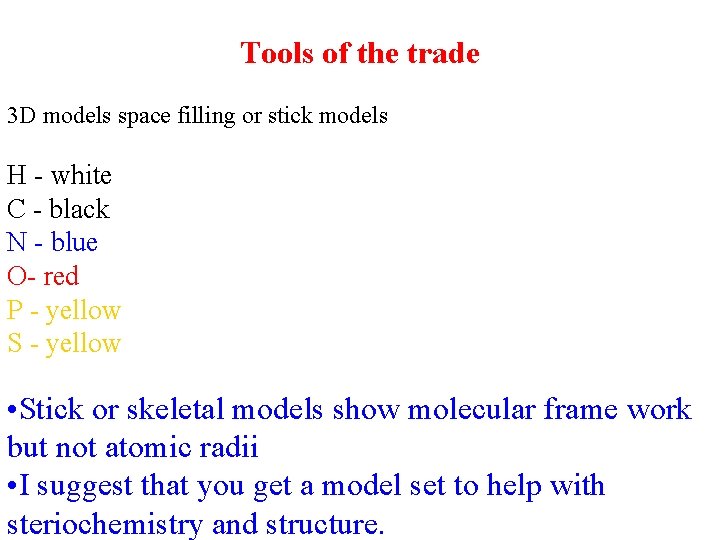
Tools of the trade 3 D models space filling or stick models H - white C - black N - blue O- red P - yellow S - yellow • Stick or skeletal models show molecular frame work but not atomic radii • I suggest that you get a model set to help with steriochemistry and structure.

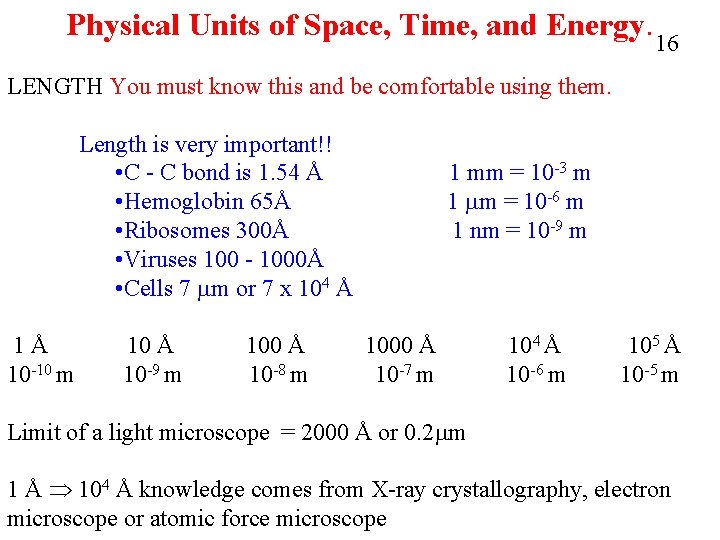
Physical Units of Space, Time, and Energy. 16 LENGTH You must know this and be comfortable using them. Length is very important!! • C - C bond is 1. 54 Å • Hemoglobin 65Å • Ribosomes 300Å • Viruses 100 - 1000Å • Cells 7 mm or 7 x 104 Å 1Å 10 -10 m 10 Å 10 -9 m 100 Å 10 -8 m 1 mm = 10 -3 m 1 mm = 10 -6 m 1 nm = 10 -9 m 1000 Å 10 -7 m 104 Å 10 -6 m 105 Å 10 -5 m Limit of a light microscope = 2000 Å or 0. 2 mm 1 Å 104 Å knowledge comes from X-ray crystallography, electron microscope or atomic force microscope

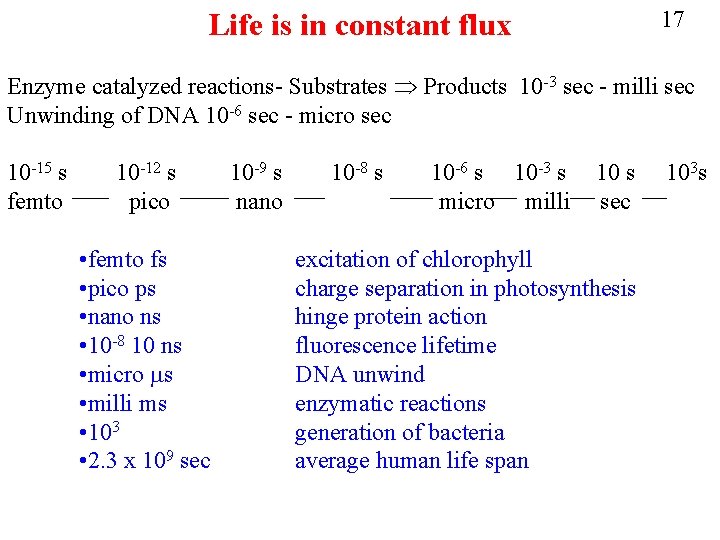
17 Life is in constant flux Enzyme catalyzed reactions- Substrates Products 10 -3 sec - milli sec Unwinding of DNA 10 -6 sec - micro sec 10 -15 s femto 10 -12 s pico • femto fs • pico ps • nano ns • 10 -8 10 ns • micro ms • milli ms • 103 • 2. 3 x 109 sec 10 -9 s nano 10 -8 s 10 -6 s 10 -3 s micro milli 10 s sec excitation of chlorophyll charge separation in photosynthesis hinge protein action fluorescence lifetime DNA unwind enzymatic reactions generation of bacteria average human life span 103 s

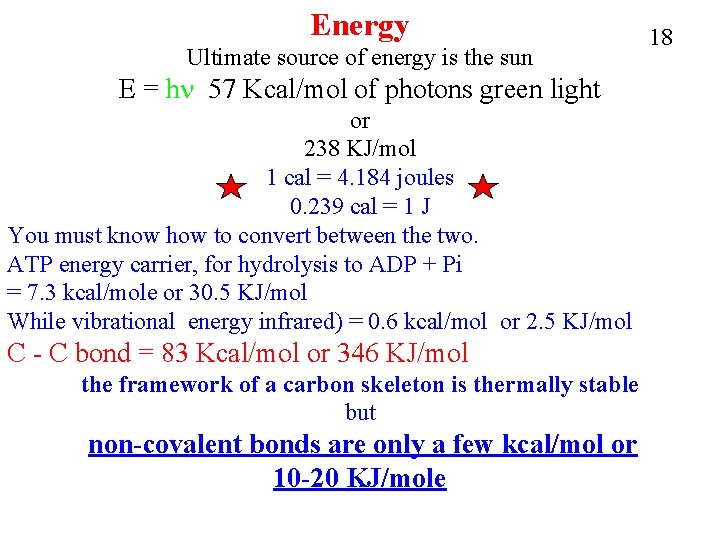
Energy Ultimate source of energy is the sun E = hn 57 Kcal/mol of photons green light or 238 KJ/mol 1 cal = 4. 184 joules 0. 239 cal = 1 J You must know how to convert between the two. ATP energy carrier, for hydrolysis to ADP + Pi = 7. 3 kcal/mole or 30. 5 KJ/mol While vibrational energy infrared) = 0. 6 kcal/mol or 2. 5 KJ/mol C - C bond = 83 Kcal/mol or 346 KJ/mol the framework of a carbon skeleton is thermally stable but non-covalent bonds are only a few kcal/mol or 10 -20 KJ/mole 18

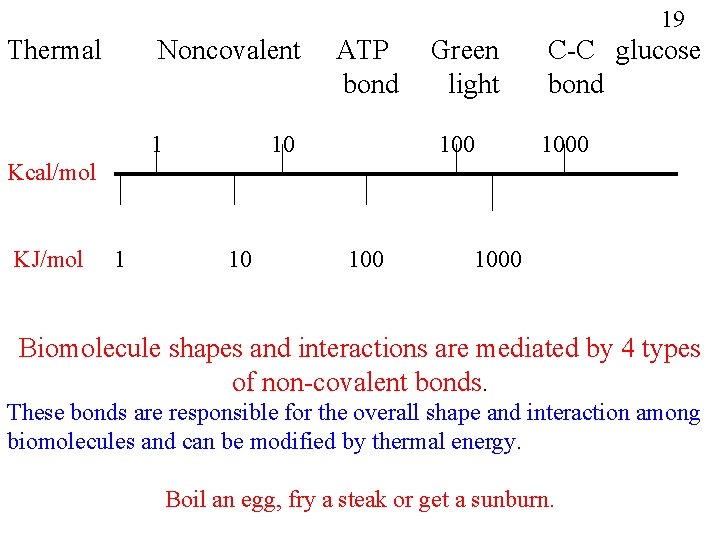
19 Thermal Noncovalent 1 ATP bond 10 Green light 100 C-C glucose bond 1000 Kcal/mol KJ/mol 1 10 1000 Biomolecule shapes and interactions are mediated by 4 types of non-covalent bonds. These bonds are responsible for the overall shape and interaction among biomolecules and can be modified by thermal energy. Boil an egg, fry a steak or get a sunburn.

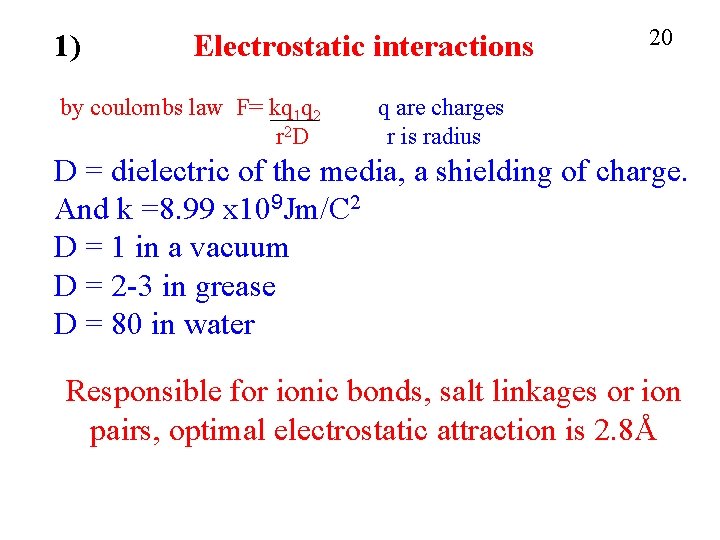
1) Electrostatic interactions by coulombs law F= kq 1 q 2 r 2 D 20 q are charges r is radius D = dielectric of the media, a shielding of charge. And k =8. 99 x 109 Jm/C 2 D = 1 in a vacuum D = 2 -3 in grease D = 80 in water Responsible for ionic bonds, salt linkages or ion pairs, optimal electrostatic attraction is 2. 8Å

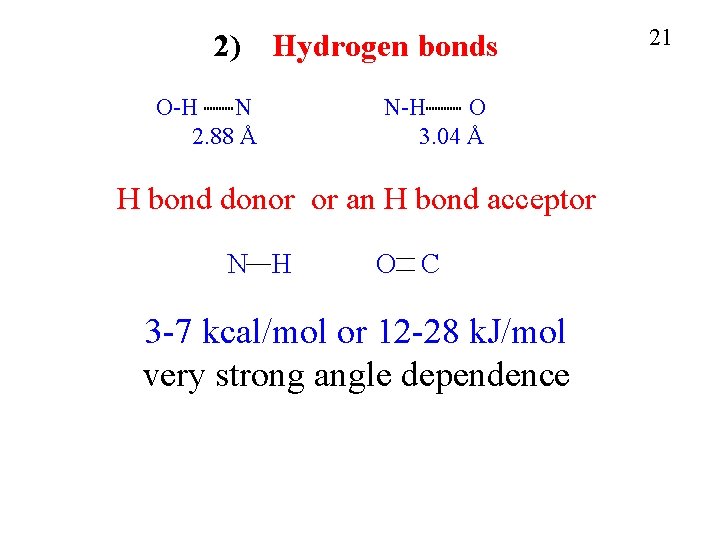
2) Hydrogen bonds O-H N 2. 88 Å N-H O 3. 04 Å H bond donor or an H bond acceptor N H O C 3 -7 kcal/mol or 12 -28 k. J/mol very strong angle dependence 21

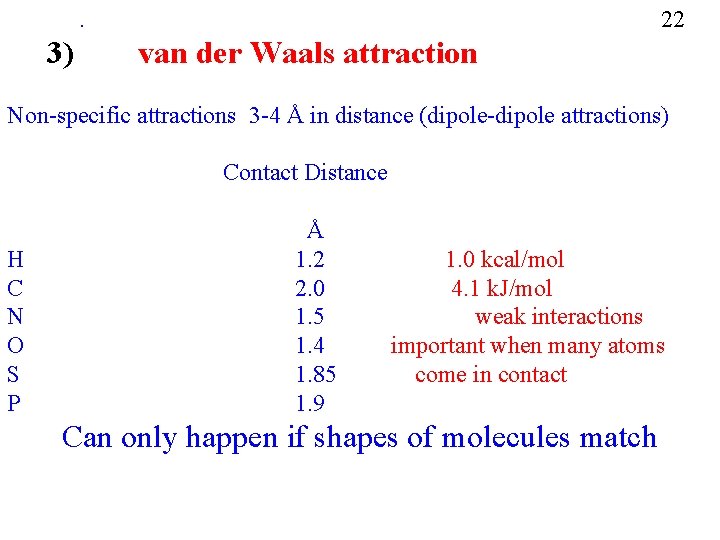
22 . 3) van der Waals attraction Non-specific attractions 3 -4 Å in distance (dipole-dipole attractions) Contact Distance H C N O S P Å 1. 2 2. 0 1. 5 1. 4 1. 85 1. 9 1. 0 kcal/mol 4. 1 k. J/mol weak interactions important when many atoms come in contact Can only happen if shapes of molecules match

Steric complementarity 23 • Occurs when large numbers of atoms are in contact Specificity When there is a large affinity for a unique molecule to bind to another a) antibodies b) enzyme substrate c) restriction enzymes

Dielectric effect hexane benzene diethyl ether CHCl 3 acetone Ethanol methanol H 2 O HCN D 1. 9 2. 3 4. 3 5. 1 21. 4 24 33 80 116 24 H 2 O is an excellent solvent and dissolves a large array of polar molecules. However, it also weakens ionic and hydrogen bonds Therefore, biological systems sometimes exclude H 2 O to form maximal strength bonds!!

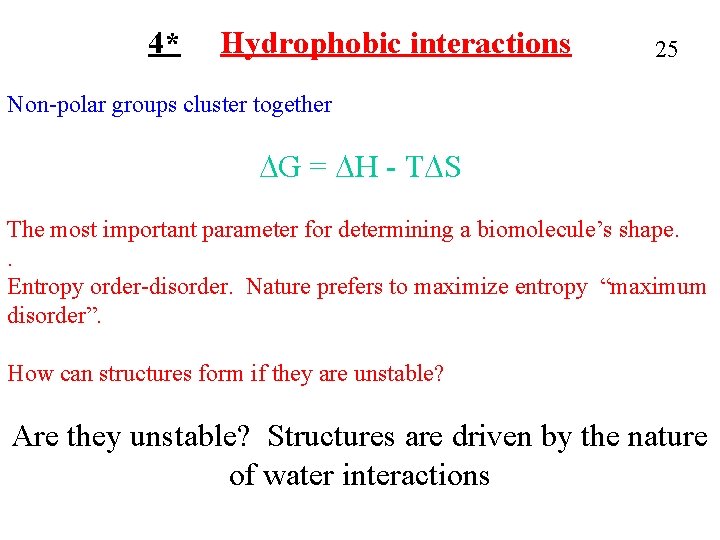
4* Hydrophobic interactions 25 Non-polar groups cluster together DG = DH - TDS The most important parameter for determining a biomolecule’s shape. . Entropy order-disorder. Nature prefers to maximize entropy “maximum disorder”. How can structures form if they are unstable? Are they unstable? Structures are driven by the nature of water interactions