What did we learn from the WOEST Trial





- Slides: 15

What did we learn from the WOEST Trial? Are we read to drop aspirin from triple therapy? Sunil V. Rao MD

Disclosures n Consultant l n Research funding l n Medtronic, Merck, AZ, Boehringer Ingelheim Bellerophon Off-label uses may be discussed

Are we ready to drop aspirin? n Scope of the problem – how many patients are potentially candidates for triple therapy? n Risks of triple therapy n What did we learn from WOEST? n Are we ready to drop aspirin?

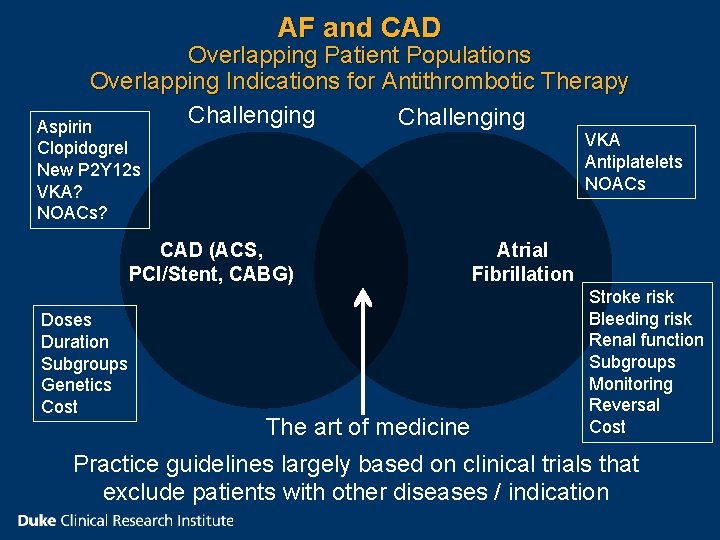
AF and CAD Overlapping Patient Populations Overlapping Indications for Antithrombotic Therapy Challenging Aspirin VKA Antiplatelets NOACs Clopidogrel New P 2 Y 12 s VKA? NOACs? CAD (ACS, PCI/Stent, CABG) Doses Duration Subgroups Genetics Cost The art of medicine Atrial Fibrillation Stroke risk Bleeding risk Renal function Subgroups Monitoring Reversal Cost Practice guidelines largely based on clinical trials that exclude patients with other diseases / indication

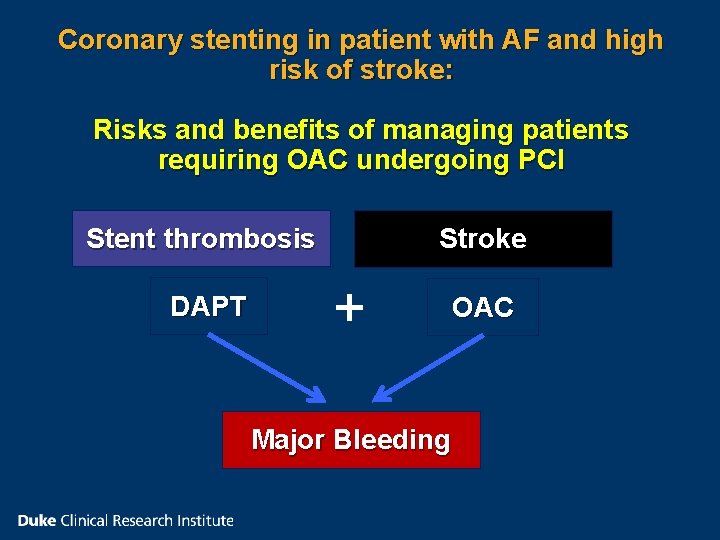
Coronary stenting in patient with AF and high risk of stroke: Risks and benefits of managing patients requiring OAC undergoing PCI Stroke Stent thrombosis DAPT + OAC Major Bleeding

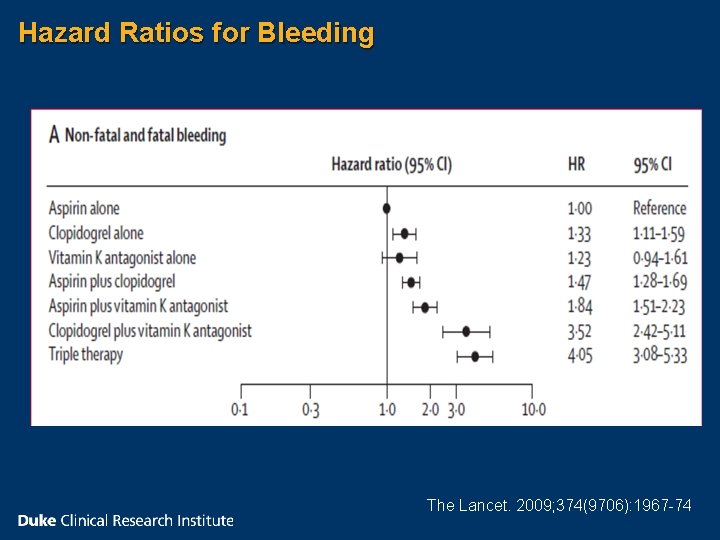
• Over 40, 000 patients • Registries from Denmark • 2000 -2005 • Mean Follow-up 476 days • 4. 6% of patients were admitted to hospital with bleeding The Lancet. 2009; 374(9706): 1967 -74

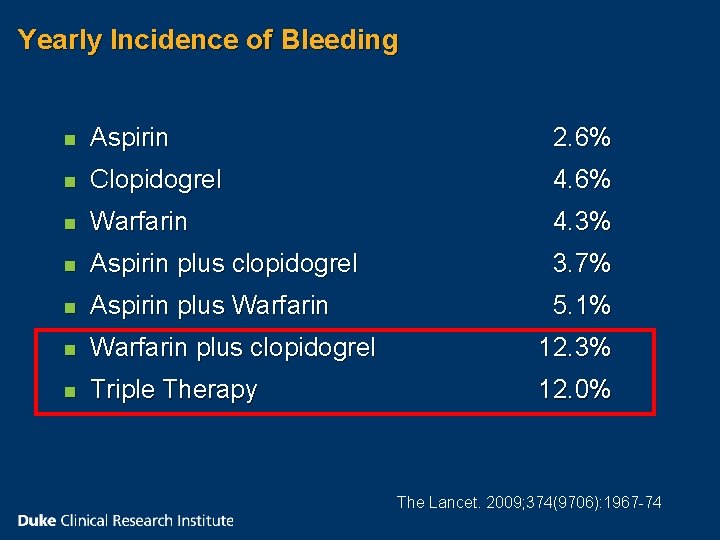
Yearly Incidence of Bleeding n Aspirin 2. 6% n Clopidogrel 4. 6% n Warfarin 4. 3% n Aspirin plus clopidogrel 3. 7% n Aspirin plus Warfarin 5. 1% n Warfarin plus clopidogrel 12. 3% n Triple Therapy 12. 0% The Lancet. 2009; 374(9706): 1967 -74

Hazard Ratios for Bleeding The Lancet. 2009; 374(9706): 1967 -74

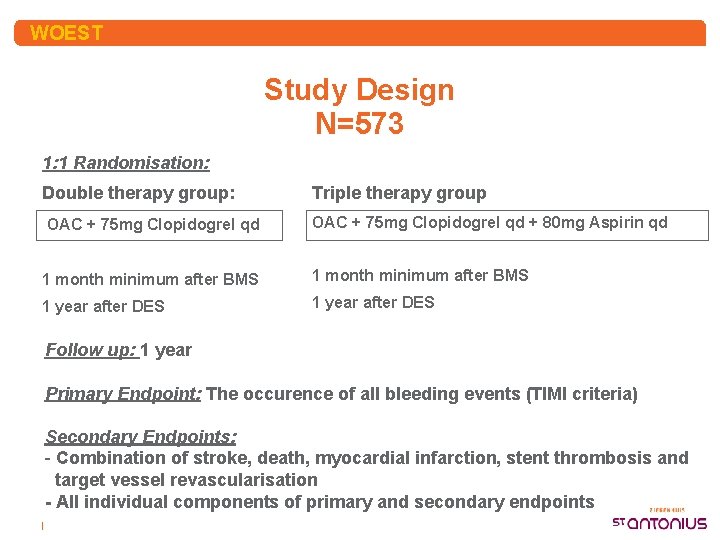
WOEST Study Design N=573 1: 1 Randomisation: Double therapy group: OAC + 75 mg Clopidogrel qd Triple therapy group OAC + 75 mg Clopidogrel qd + 80 mg Aspirin qd 1 month minimum after BMS 1 year after DES Follow up: 1 year Primary Endpoint: The occurence of all bleeding events (TIMI criteria) Secondary Endpoints: - Combination of stroke, death, myocardial infarction, stent thrombosis and target vessel revascularisation - All individual components of primary and secondary endpoints |

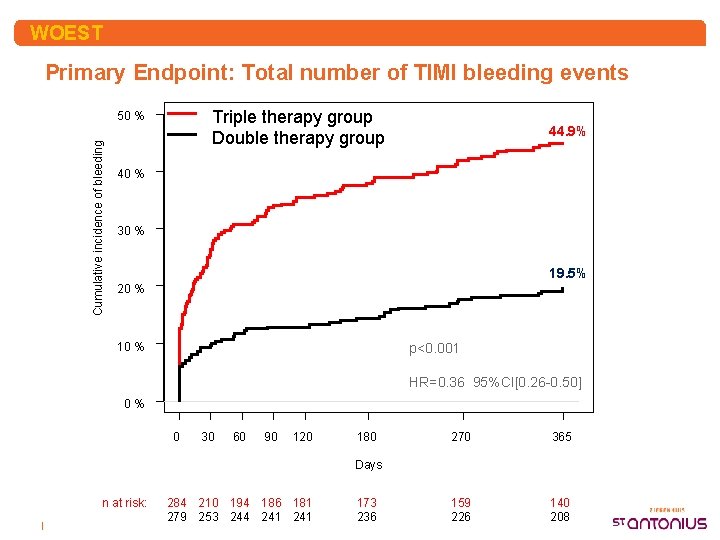
WOEST Primary Endpoint: Total number of TIMI bleeding events Triple therapy group Double therapy group Cumulative incidence of bleeding 50 % 44. 9% 40 % 30 % 19. 5% 20 % 10 % p<0. 001 HR=0. 36 95%CI[0. 26 -0. 50] 0% 0 30 60 90 120 180 270 365 159 226 140 208 Days n at risk: | 284 210 194 186 181 279 253 244 241 173 236

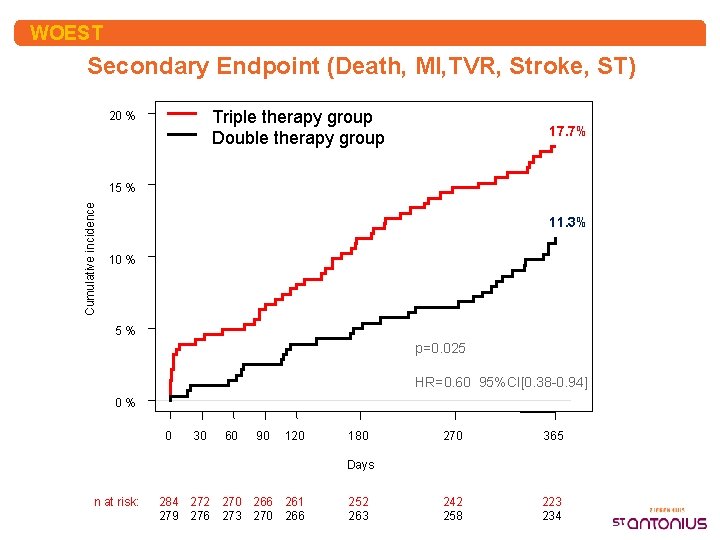
WOEST Secondary Endpoint (Death, MI, TVR, Stroke, ST) Triple therapy group Double therapy group 20 % 17. 7% Cumulative incidence 15 % 11. 3% 10 % 5% p=0. 025 HR=0. 60 95%CI[0. 38 -0. 94] 0% 0 30 60 90 120 180 270 365 242 258 223 234 Days n at risk: 284 272 270 266 261 279 276 273 270 266 252 263

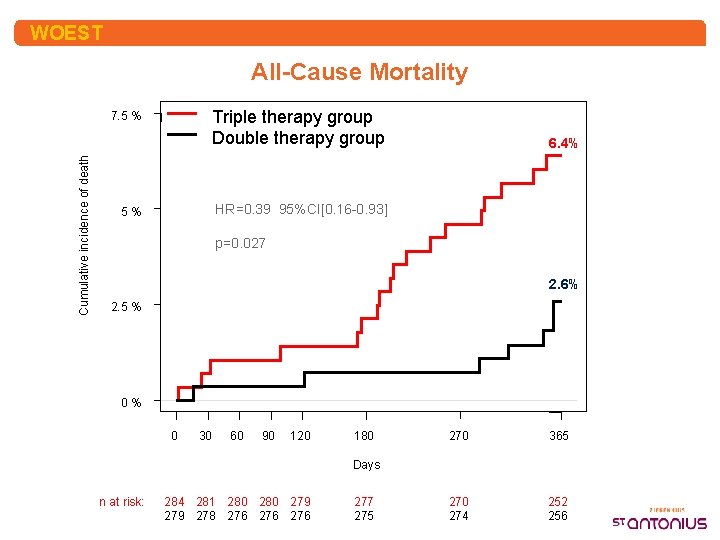
WOEST All-Cause Mortality Triple therapy group Double therapy group Cumulative incidence of death 7. 5 % 6. 4% HR=0. 39 95%CI[0. 16 -0. 93] 5% p=0. 027 2. 6% 2. 5 % 0% 0 30 60 90 120 180 270 365 270 274 252 256 Days n at risk: 284 281 280 279 278 276 276 277 275

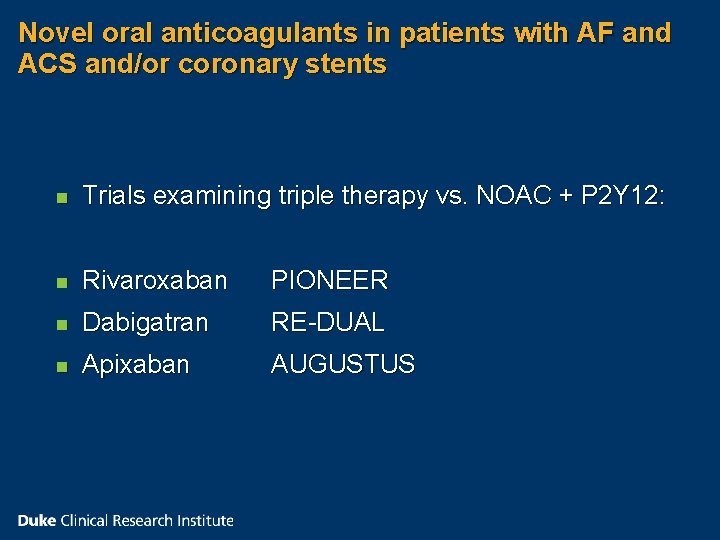
Novel oral anticoagulants in patients with AF and ACS and/or coronary stents n Trials examining triple therapy vs. NOAC + P 2 Y 12: n Rivaroxaban PIONEER n Dabigatran RE-DUAL n Apixaban AUGUSTUS

Are we ready to drop aspirin from triple therapy? n Goals of therapy in patients requiring (N)OAC and DAPT: l l Reduce the risk for stroke and/or valve thrombosis Minimize bleeding risk n WOEST trial suggests that with contemporary stents, aspirin can be dropped from therapy with no penalty and maybe decreased mortality n But…RCTs with NOACs are ongoing n Until these are available, would only consider dropping aspirin in patients at highest risk for bleeding l Older age, anemia, known bleeding source

Duke Univ. Medical Center Thank you. Duke Clinical Research Institute