What are we going to talk about What


What are we going to talk about? üWhat is Acid Rain? üHow is Acid Rain caused? üHow can we minimize it ?

What is Acid Rain ? Do you know? • Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low p. H). It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxides, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970 s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain cause paint to peel , corrosion of steel structures such as bridges, and erosion of stone statues.
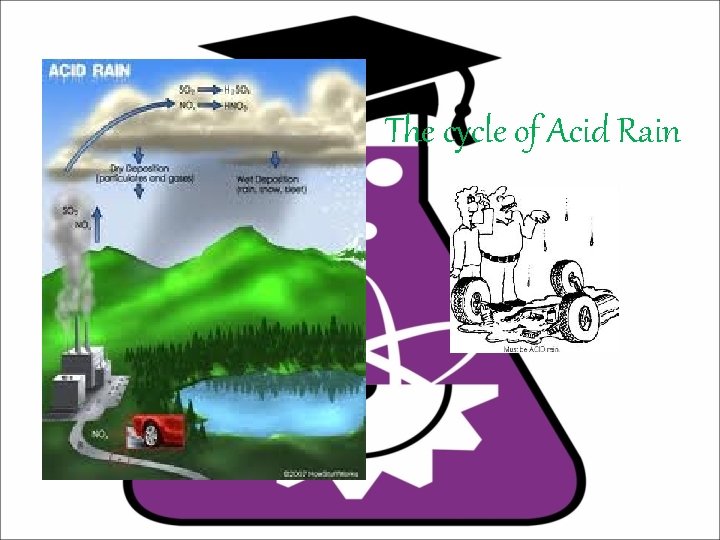
The cycle of Acid Rain

How is Acid Rain caused? • Acid deposition can occur via natural sources like volcanoes but it is mainly caused by the release of sulfur dioxide and nitrogen oxide during fossil fuel combustion. When these gases are discharged into the atmosphere they react with the water, oxygen, and other gases already present there to form sulfuric acid, ammonium nitrate, and nitric acid. Although it was discovered in the 1800 s, acid deposition did not gain significant public attention until the 1960 s and the term acid rain was coined in 1972. Public attention further increased in the 1970 s when the New York Times published reports about problems occurring in the Hubbard Brook Experimental Forest in New Hampshire.
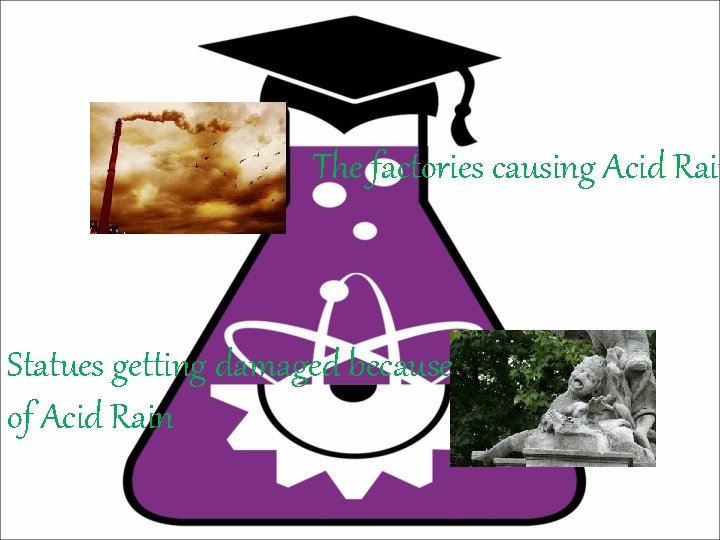
The factories causing Acid Rain Statues getting damaged because of Acid Rain

How can we minimize it ? • Acid rain can be prevented by reducing emissions that come from the mining industries, burning of fuels for electrical power generation, oil operations, and reducing fuel emissions from cars and trucks. Reduce our use of products that produce chlorofluorocarbons that are released into the atmosphere by actively conserving the use of your air conditioning. Become energy wise by conserving energy through the use of fluorescent lights, closing off rooms you do not use, and use washers, dryers, and dishwashers only when full. Last but not lease please recycle packaging and other disposable products.
Minimize the use of electrical objects and other stuff……… Earth is in our hands , protect it.

1960 2012 You can see the difference between 1960 and 2012 , the colour changed because of acid rain.

Sulfur dioxide reacts with water to form sulfurous acid. SO 2(g) + H 2 O(l) -> H 2 SO 3(aq) Substances in the upper atmosphere then catalyze the reaction between sulfurous acid and oxygen to form sulfuric acid. 2 H 2 SO 3(aq) + O 2(g) -> 2 H 2 SO 4(aq) Similarly, nitrogen dioxide reacts with water to form a mixture of nitric acid and nitrous acid. 2 NO 2(g) + H 2 O(l) -> HNO 3(aq) + HNO 2(aq) Substances in the atmosphere then catalyze the reaction between nitrous acid and oxygen causing the formation of more nitric acid. 2 HNO 2(aq) + O 2(g) -> 2 HNO 3(aq) Limestone and Marble Ca. CO 3(s) + H 2 SO 4(aq) -> CO 2(g) + H 2 O(l) + Ca(NO 3)2(aq) Metal Corrosion Metal + Acid -> Salt + Water i. e. Fe(II)(s) + H 2 SO 4(aq) -> Fe. SO 4(aq) + 2 H+
- Slides: 10