What are we doing today Demonstration of decanting

















- Slides: 34

What are we doing today? • Demonstration of decanting • Mandatory experiment on evaporation • Mandatory experiment on filtration

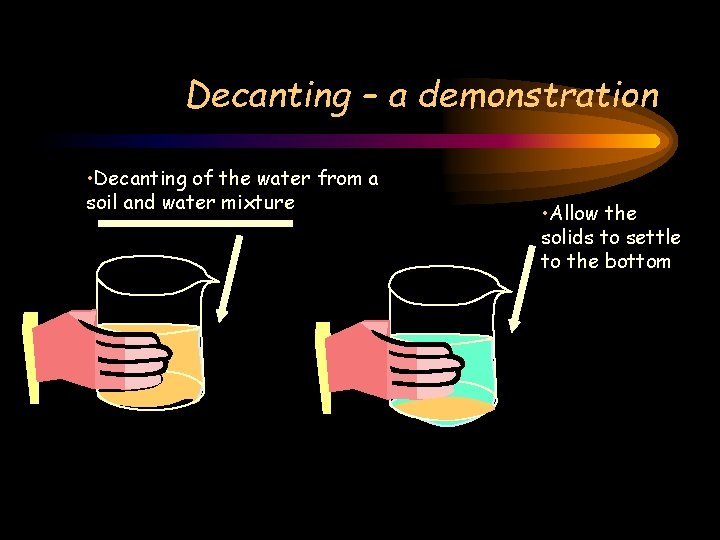
Decanting – a demonstration • Decanting of the water from a soil and water mixture • Allow the solids to settle to the bottom

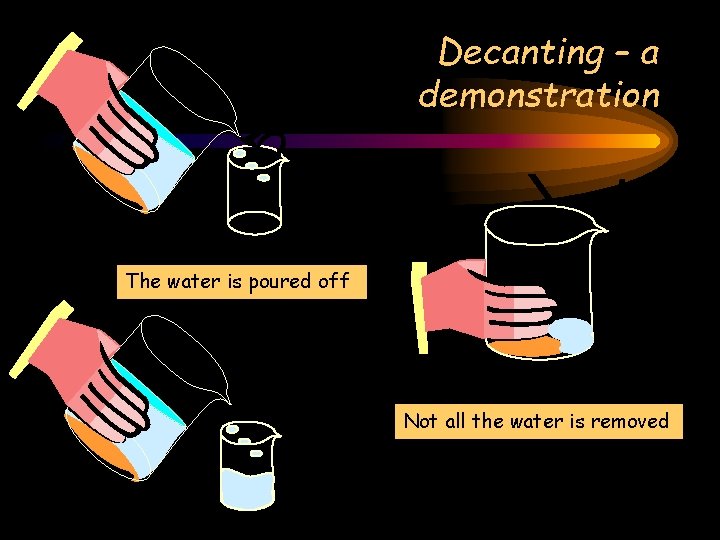
Decanting – a demonstration The water is poured off Not all the water is removed

Mandatory Experiment 11 b – To separate salt and water using evaporation • AIM – to use evaporation to separate the water from a mixture of salt ad water BUT • WHAT IS EVAPORATION?

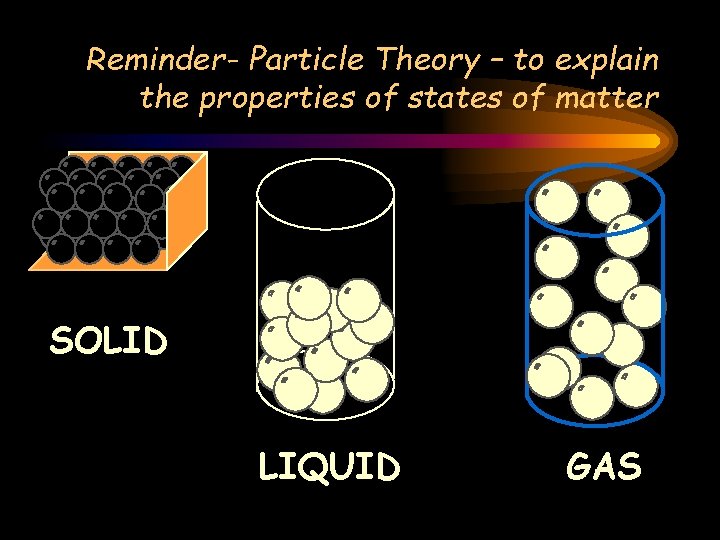
Reminder- Particle Theory – to explain the properties of states of matter SOLID LIQUID GAS

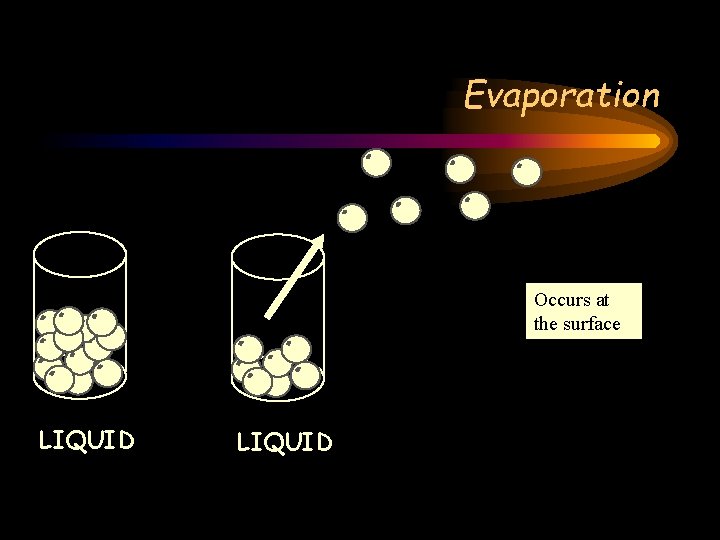
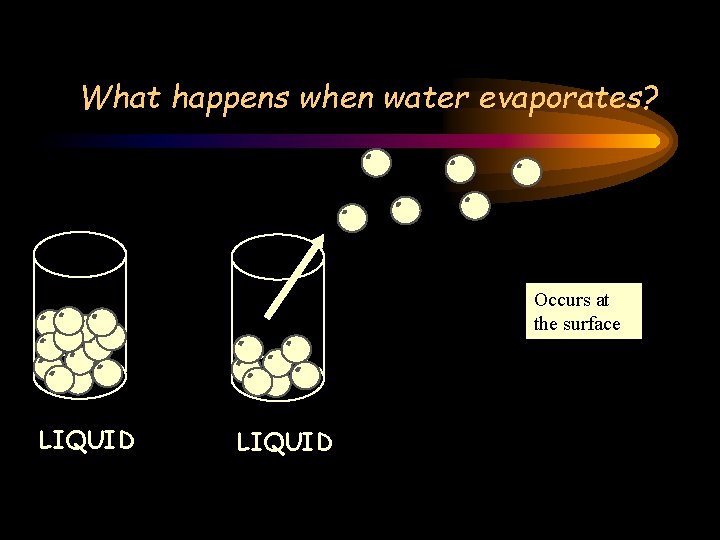
Evaporation Occurs at the surface LIQUID

Mandatory Experiment 11 b – To separate salt and water using evaporation • Evaporation of the water from a salt and water mixture

Mandatory Experiment 11 b – To separate salt and water using evaporation • Evaporation of the water from a salt and water mixture RESULT – salt is collected

Mandatory Experiment 11 a – To separate soil and water using filtration Filter paper Funnel Conical flask A mixture of soil and water

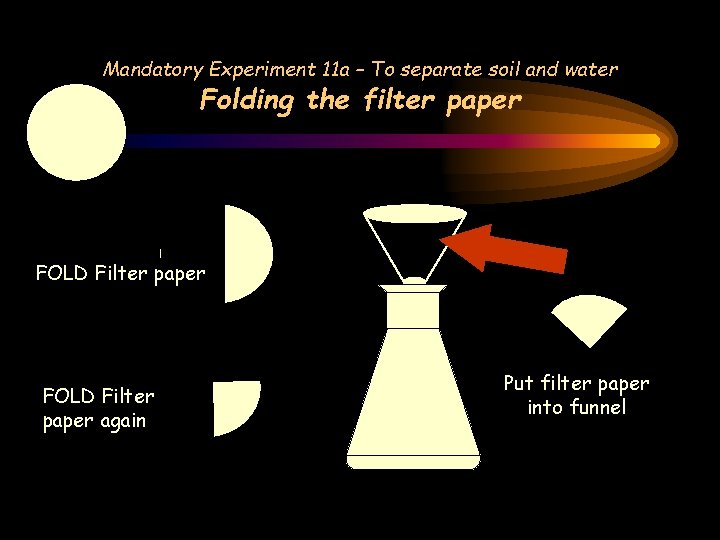
Mandatory Experiment 11 a – To separate soil and water Folding the filter paper FOLD Filter paper again Put filter paper into funnel

Mandatory Experiment 11 a – To separate soil and water using filtration Pour the mixture of soil and water into the funnel

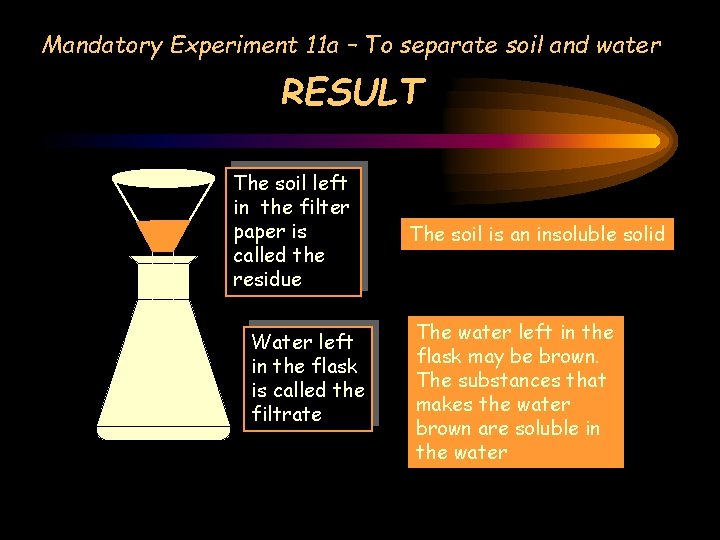
Mandatory Experiment 11 a – To separate soil and water RESULT The soil left in the filter paper is called the residue Water left in the flask is called the filtrate The soil is an insoluble solid The water left in the flask may be brown. The substances that makes the water brown are soluble in the water

Separation Techniques - Continued

What woulld happen if you had no clean water – say in a desert island? • All you had was SEA water How could you obtain pure fresh water? • Using the technique of Evaporation of the water from a salt and water mixture – no good as you loose the water

Separation Techniques - Continued YOU NEED A NEW TECHNIQUE - CALLED DISTILLATION

What happens when water evaporates? Occurs at the surface LIQUID

What happens when gas hits something cold? LIQUID ?

REM EMB ER – ‘chan ges o f sta te’ Water – a good example of a material that can exist in different states ICE Solid liquid WATER gas STEAM

st ’ e at of example of a material Water – a good s e g n exist in different states that can a ch ‘ – R E B MELTING M E R BOILING Solid liquid FREEZING gas CONDENSING

What happens when gas hits something cold? It turns back to water LIQUID It condenses

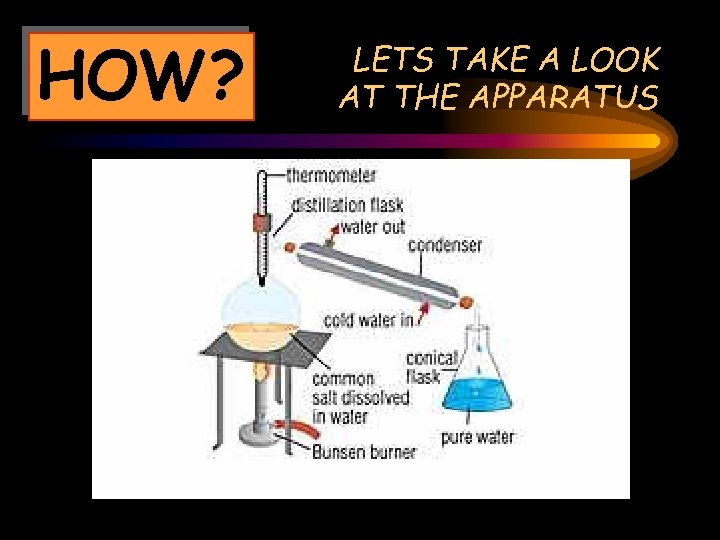
HOW? LETS TAKE A LOOK AT THE APPARATUS

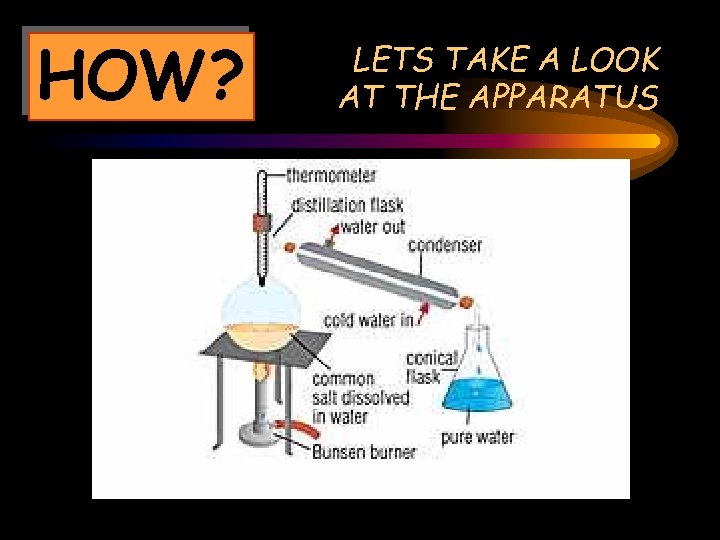
What happens? The mixture of salt and water (in the distillation flask) is heated by the bunsen burner The condenser turns the steam back to water and the water is collected in the conical flask The water turns to steam The steam rises up and comes in contact with the condenser

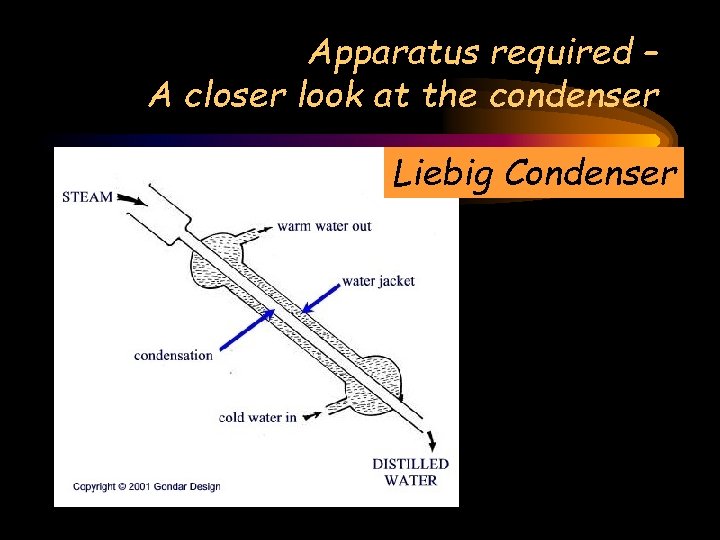
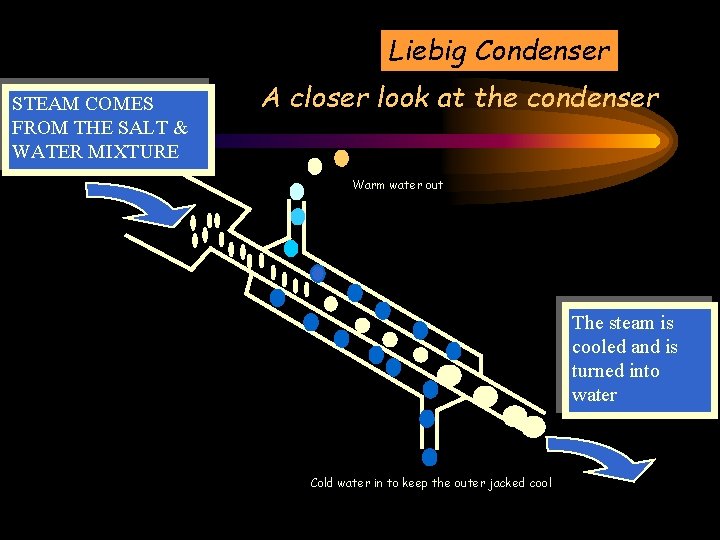
Apparatus required – A closer look at the condenser Liebig Condenser

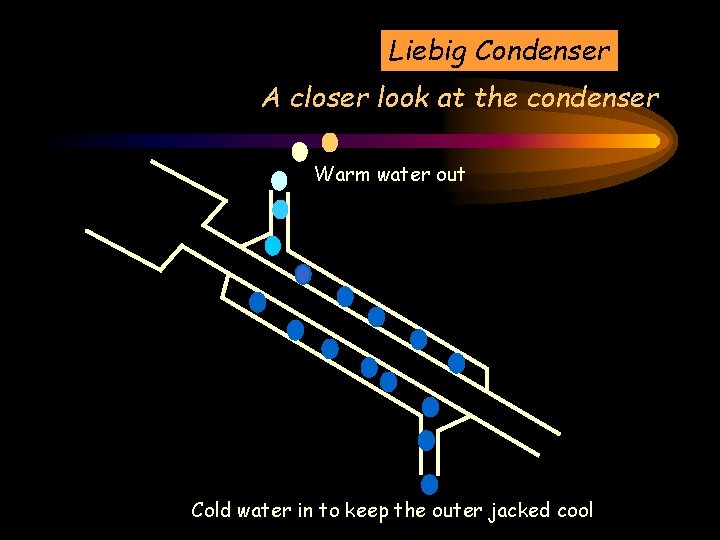
Liebig Condenser A closer look at the condenser Warm water out Cold water in to keep the outer jacked cool

Liebig Condenser STEAM COMES FROM THE SALT & WATER MIXTURE A closer look at the condenser Warm water out The steam is cooled and is turned into water Cold water in to keep the outer jacked cool

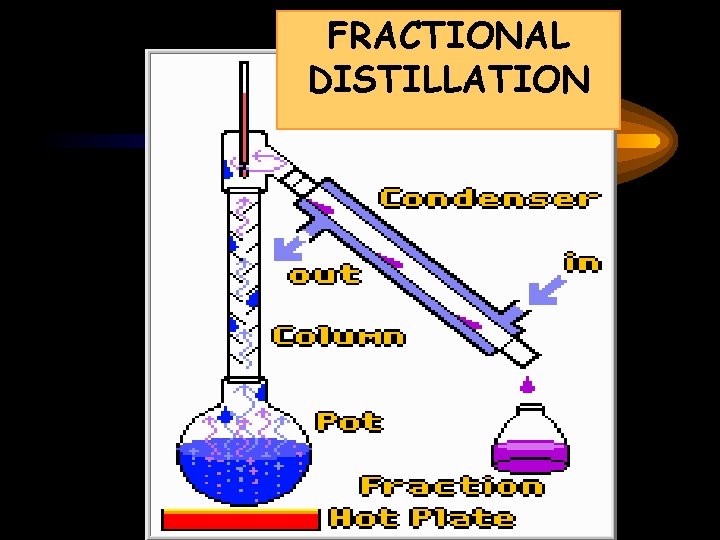
FRACTIONAL DISTILLATION

Pharamaceutical

Oil refinery

The manufacture of perfume

Distillation of wine to make brandy

What are we doing today? • Mandatory experiment on DISTILLATION

Mandatory Experiment 11 C – To separate salt and water using distillation • Evaporation of the water from a salt and water mixture BUT THIS TIME WE WANT TO SAVE THE WATER …and we use different apparatus

HOW? LETS TAKE A LOOK AT THE APPARATUS

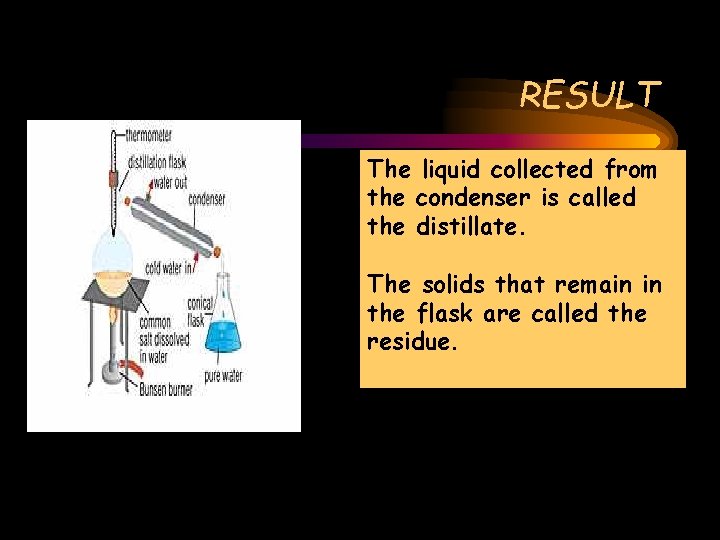
RESULT The liquid collected from the condenser is called the distillate. The solids that remain in the flask are called the residue.