What are they There are different definitions for







- Slides: 18

What are they? There are different definitions for acids and bases depending on the circumstances. Acid—produces hydrogen ions (H+) in solution Bases—produces hydroxide ions (OH-) in solution

1. 2. 3. 4. 5. 6. 7. Acids react with carbonates to produce carbon dioxide gas. change pink phenolphthalein to colourless make litmus paper turn red or stay red make bromothymol blue turn yellow taste sour have a p. H below 7 react with most metals producing hydrogen gas

Bases 1. don’t react with carbonates to produce 2. 3. 4. 5. 6. carbon dioxide gas. change colourless phenolphthalein to pink make litmus paper turn blue or stay blue make bromothymol blue stay blue taste bitter have a p. H above 7

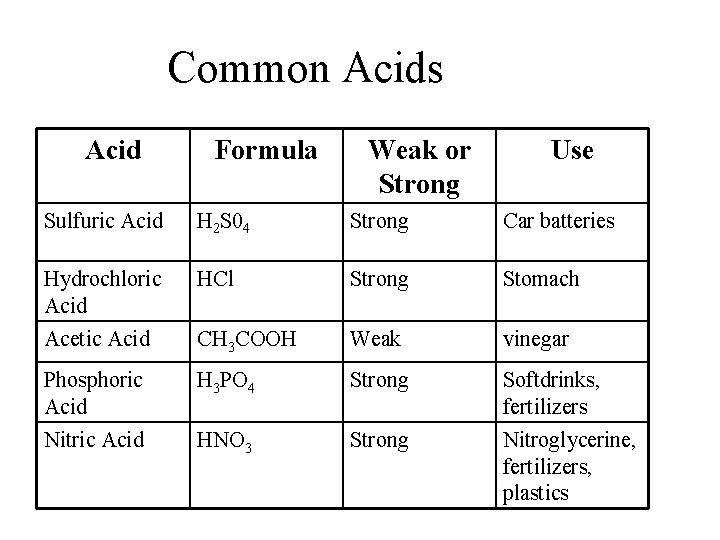
Common Acids Acid Formula Weak or Strong Use Sulfuric Acid H 2 S 04 Strong Car batteries Hydrochloric Acid HCl Strong Stomach Acetic Acid CH 3 COOH Weak vinegar Phosphoric Acid H 3 PO 4 Strong Softdrinks, fertilizers Nitric Acid HNO 3 Strong Nitroglycerine, fertilizers, plastics

Common Bases Base Formula Weak or Strong Use Ammonia NH 3 Weak Windex Sodium Hydroxide or Lye Na. OH Strong Drain Cleaners Calcium Carbonate Ca. CO 3 Weak Antacids such as Tums, Rolaids Potassium hydroxide KOH Strong Bio diesel, soaps

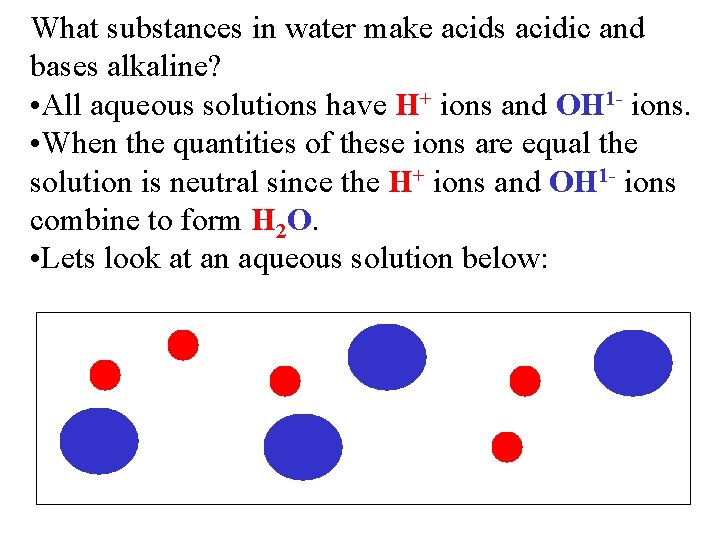
What substances in water make acids acidic and bases alkaline? • All aqueous solutions have H+ ions and OH 1 - ions. • When the quantities of these ions are equal the solution is neutral since the H+ ions and OH 1 - ions combine to form H 2 O. • Lets look at an aqueous solution below:

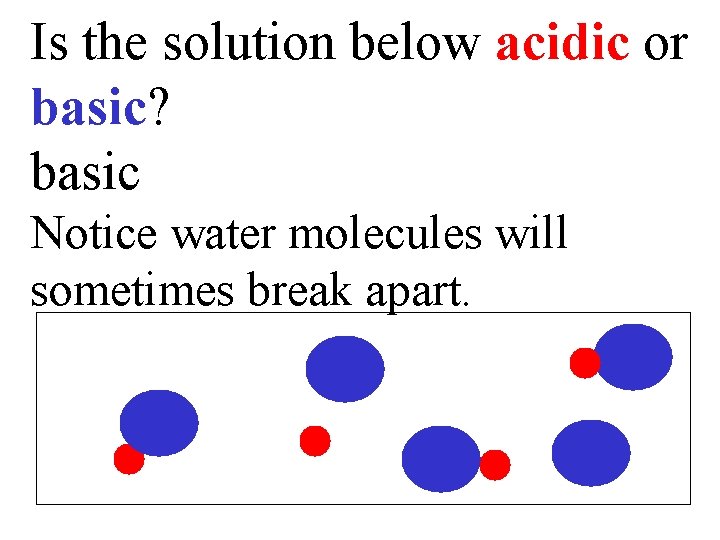
Is the solution below acidic or basic? basic Notice water molecules will sometimes break apart.

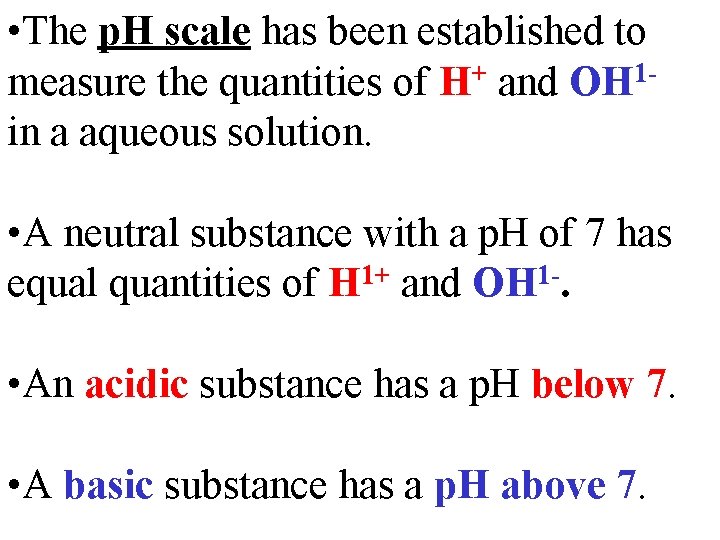
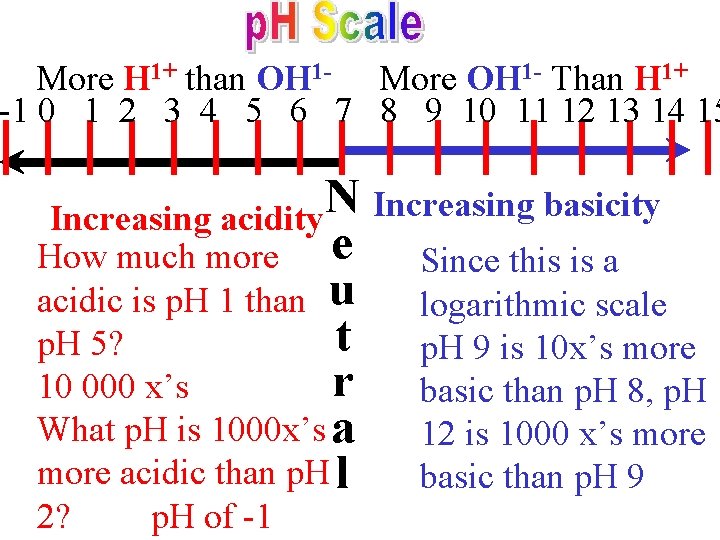
• The p. H scale has been established to measure the quantities of H+ and OH 1 in a aqueous solution. • A neutral substance with a p. H of 7 has 1+ 1 equal quantities of H and OH. • An acidic substance has a p. H below 7. • A basic substance has a p. H above 7.

More H 1+ than OH 1 - More OH 1 - Than H 1+ -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 N Increasing basicity Increasing acidity How much more e Since this is a acidic is p. H 1 than u logarithmic scale t p. H 9 is 10 x’s more p. H 5? r basic than p. H 8, p. H 10 000 x’s What p. H is 1000 x’s a 12 is 1000 x’s more acidic than p. H l basic than p. H 9 2? p. H of -1

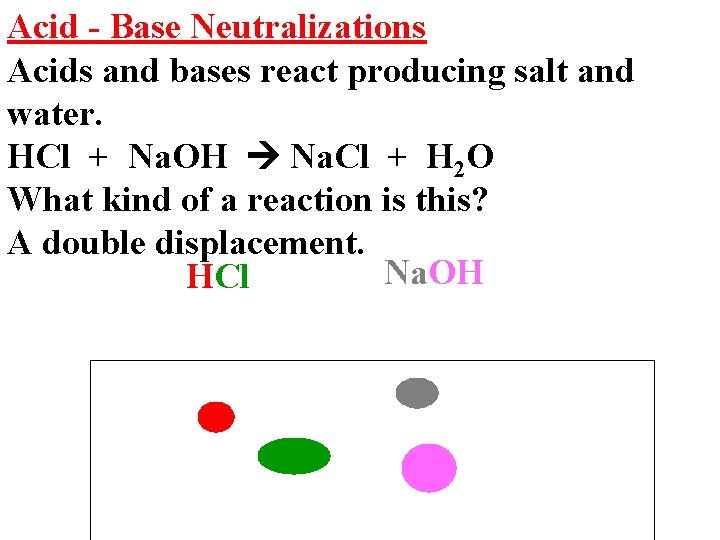
Acid - Base Neutralizations Acids and bases react producing salt and water. HCl + Na. OH Na. Cl + H 2 O What kind of a reaction is this? A double displacement. Na. OH HCl

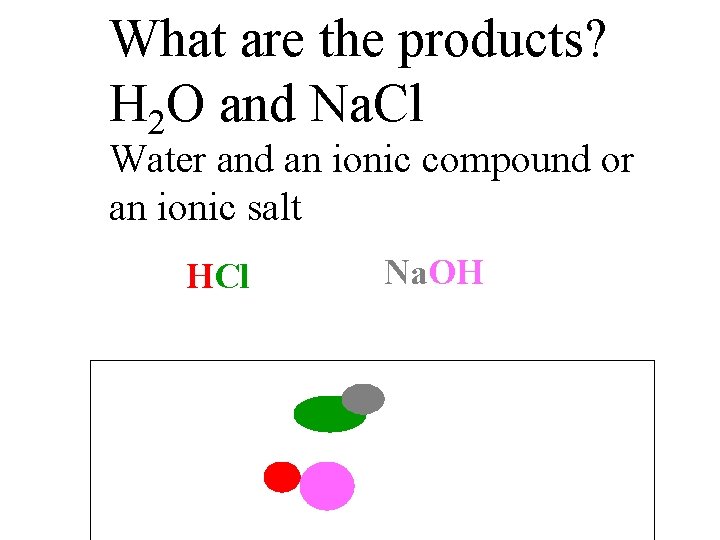
What are the products? H 2 O and Na. Cl Water and an ionic compound or an ionic salt HCl Na. OH

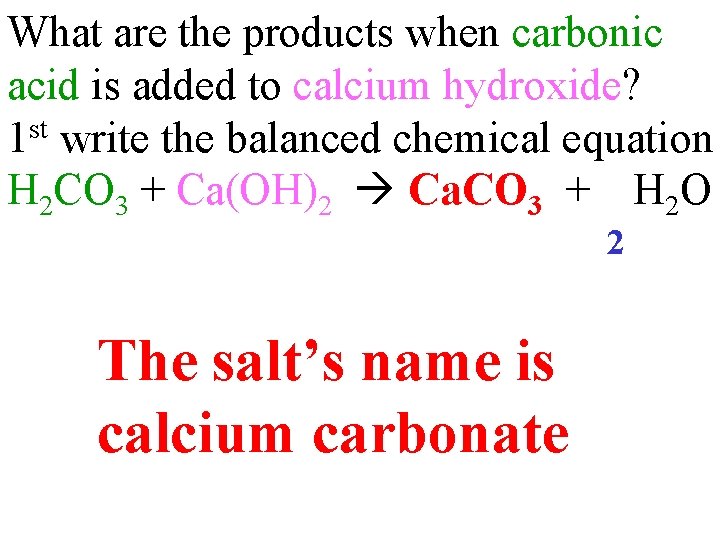
What are the products when carbonic acid is added to calcium hydroxide? 1 st write the balanced chemical equation H 2 CO 3 + Ca(OH)2 Ca. CO 3 + H 2 O 2 The salt’s name is calcium carbonate

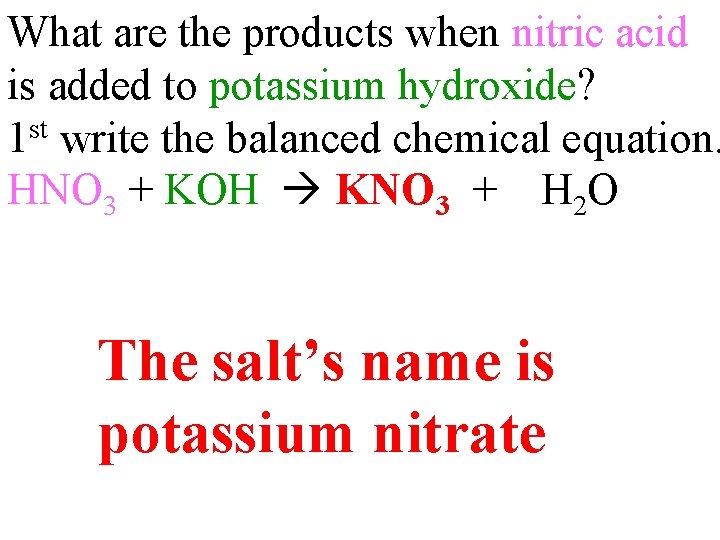
What are the products when nitric acid is added to potassium hydroxide? 1 st write the balanced chemical equation. HNO 3 + KOH KNO 3 + H 2 O The salt’s name is potassium nitrate

What are the products when chloric acid is added to lithium hydroxide? 1 st write the balanced chemical equation. HCl. O 3 + Li. OH Li. Cl. O 3 + H 2 O The salt’s name is lithium chlora

Antacids (ex. Tums or Rolaids) • Antacid tablets used to treat excess stomach acid (heartburn) are reallybases which neutralize acids. • Antacids cause a neutralization reaction in your stomach that produces salt and water.

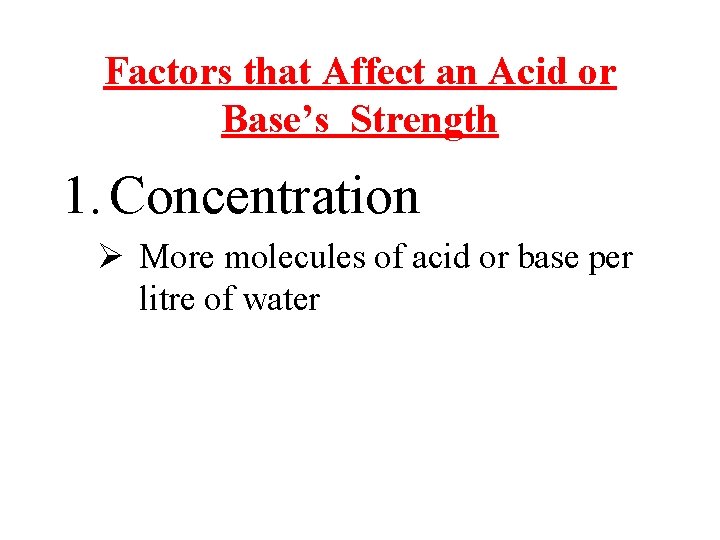
Factors that Affect an Acid or Base’s Strength 1. Concentration Ø More molecules of acid or base per litre of water

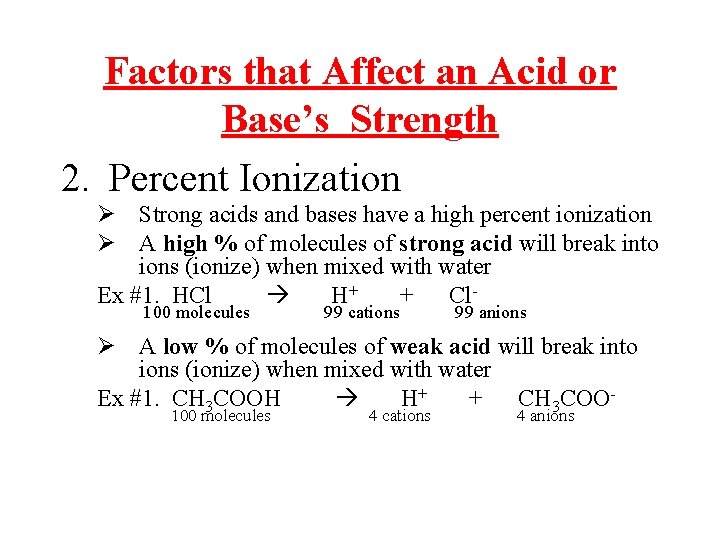
Factors that Affect an Acid or Base’s Strength 2. Percent Ionization Ø Strong acids and bases have a high percent ionization Ø A high % of molecules of strong acid will break into ions (ionize) when mixed with water Ex #1. HCl H+ + Cl 100 molecules 99 cations 99 anions Ø A low % of molecules of weak acid will break into ions (ionize) when mixed with water Ex #1. CH 3 COOH H+ + CH 3 COO 100 molecules 4 cations 4 anions

Oxides of Elements • Metal oxides in aqueous solutions tend to produce bases ØEx. Sodium reacting in water produces Sodium hydroxide • Non-metal oxides in aqueous solutions tend to produce acids ØSulfur and nitrogen oxides react with water to produce sulfuric acid and nitric acid (cause of acid precipiation)