What are the subatomic particles of an atom

What are the subatomic particles of an atom? All pupils will be able to (Baseline): Recall what atomic number represents. Most pupils will be able to (Further): Describe the structure of the atom and why atoms have no overall charge. Some pupils will be able to (Challenge): Describe the trends in atomic radius down a group and across a period.

Just how small is an atom?
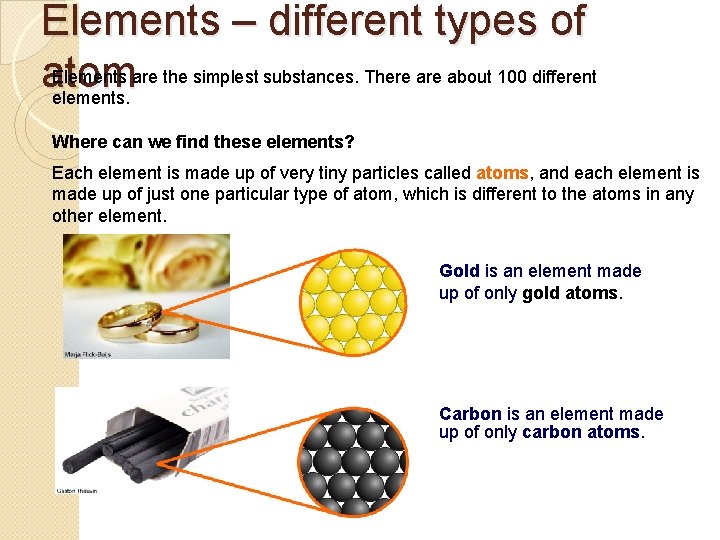
Elements – different types of Elements are the simplest substances. There about 100 different atom elements. Where can we find these elements? Each element is made up of very tiny particles called atoms, and each element is made up of just one particular type of atom, which is different to the atoms in any other element. Gold is an element made up of only gold atoms. Carbon is an element made up of only carbon atoms.
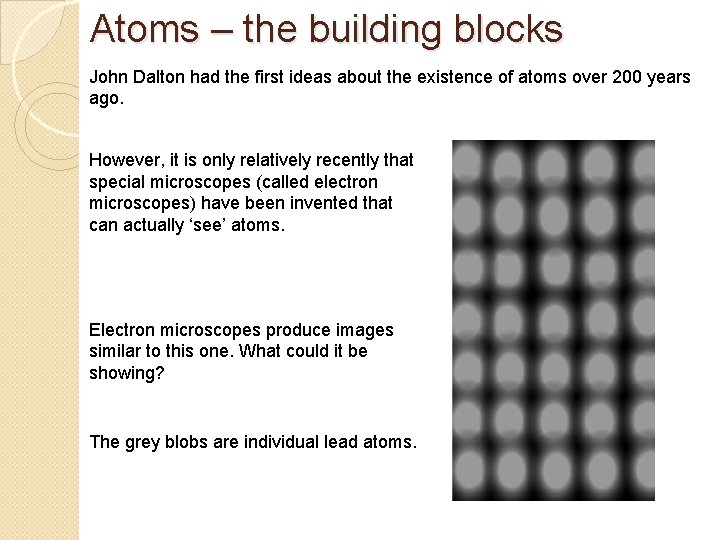
Atoms – the building blocks John Dalton had the first ideas about the existence of atoms over 200 years ago. However, it is only relatively recently that special microscopes (called electron microscopes) have been invented that can actually ‘see’ atoms. Electron microscopes produce images similar to this one. What could it be showing? The grey blobs are individual lead atoms.
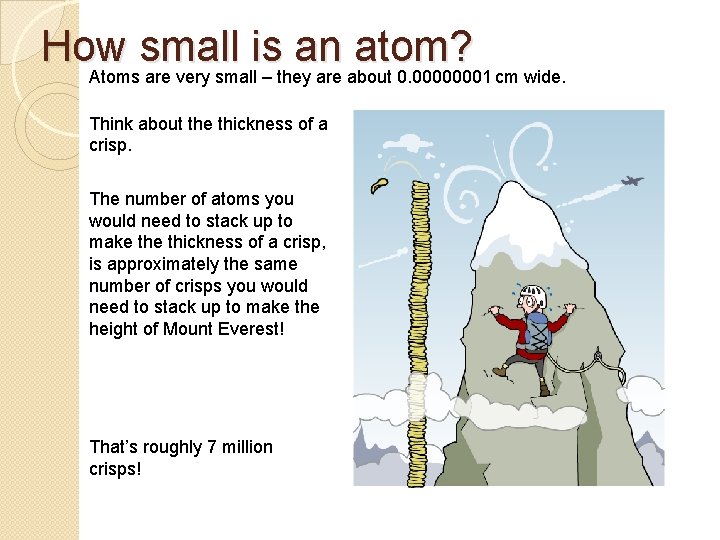
How small is an atom? Atoms are very small – they are about 0. 00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! That’s roughly 7 million crisps!
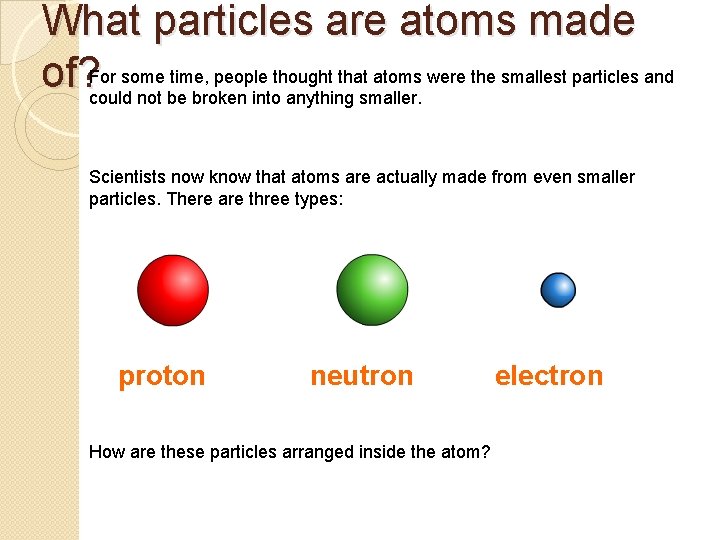
What particles are atoms made some time, people thought that atoms were the smallest particles and of? For could not be broken into anything smaller. Scientists now know that atoms are actually made from even smaller particles. There are three types: proton neutron How are these particles arranged inside the atom? electron
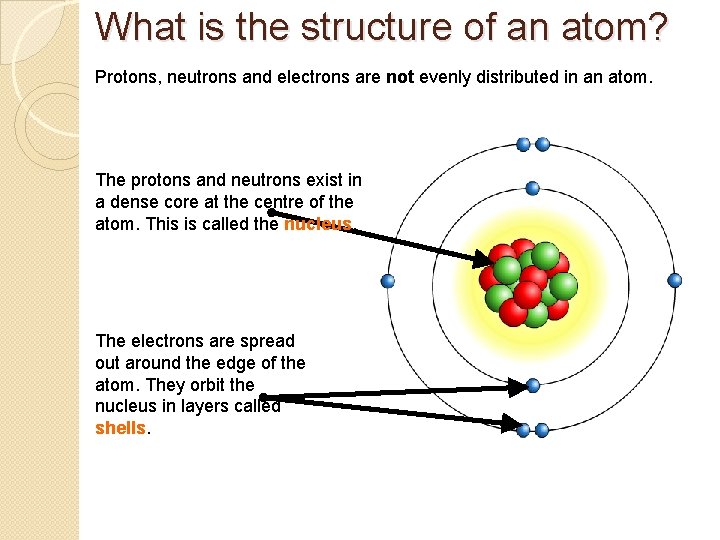
What is the structure of an atom? Protons, neutrons and electrons are not evenly distributed in an atom. The protons and neutrons exist in a dense core at the centre of the atom. This is called the nucleus. The electrons are spread out around the edge of the atom. They orbit the nucleus in layers called shells.

Labelling the atom
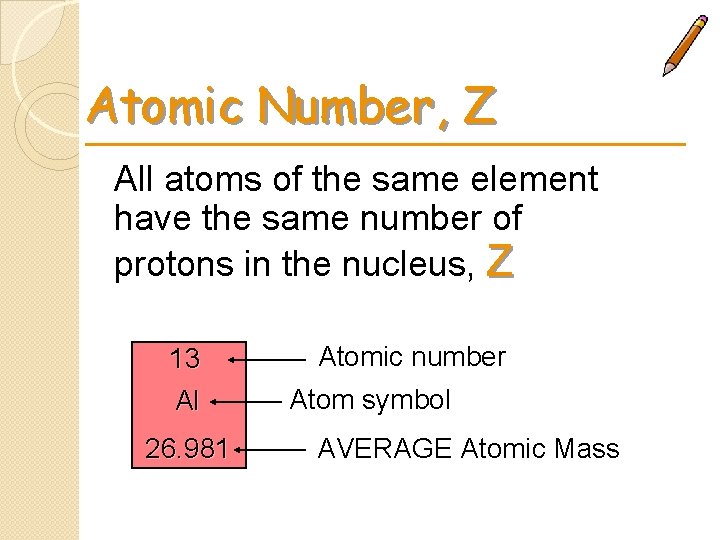
Atomic Number, Z All atoms of the same element have the same number of protons in the nucleus, Z 13 Al 26. 981 Atomic number Atom symbol AVERAGE Atomic Mass
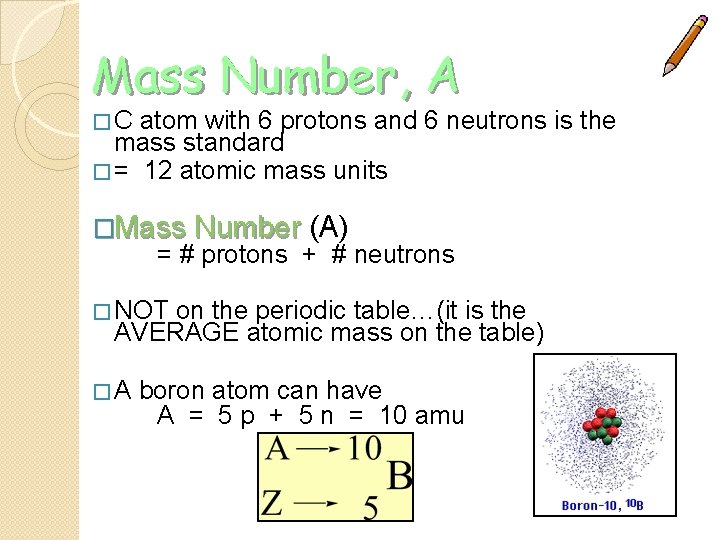
Mass Number, A � C atom with 6 protons and 6 neutrons is the mass standard � = 12 atomic mass units �Mass Number (A) = # protons + # neutrons � NOT on the periodic table…(it is the AVERAGE atomic mass on the table) � A boron atom can have A = 5 p + 5 n = 10 amu

Counting Protons, Neutrons, and Electrons �Protons = atomic number (small number from periodic table) �Neutrons = mass number – no. of protons (mass number is protons and neutrons because the mass of electrons is negligible) �Electrons = proton number ◦ If it’s an atom, the protons and electrons must be the SAME so that it is has a charge of zero, therefore NEUTRAL (equal numbers of + and -)
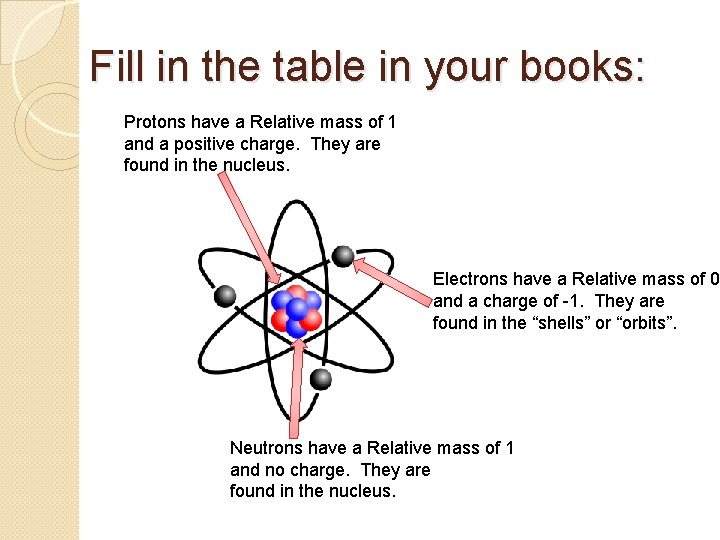
Fill in the table in your books: Protons have a Relative mass of 1 and a positive charge. They are found in the nucleus. Electrons have a Relative mass of 0 and a charge of -1. They are found in the “shells” or “orbits”. Neutrons have a Relative mass of 1 and no charge. They are found in the nucleus.
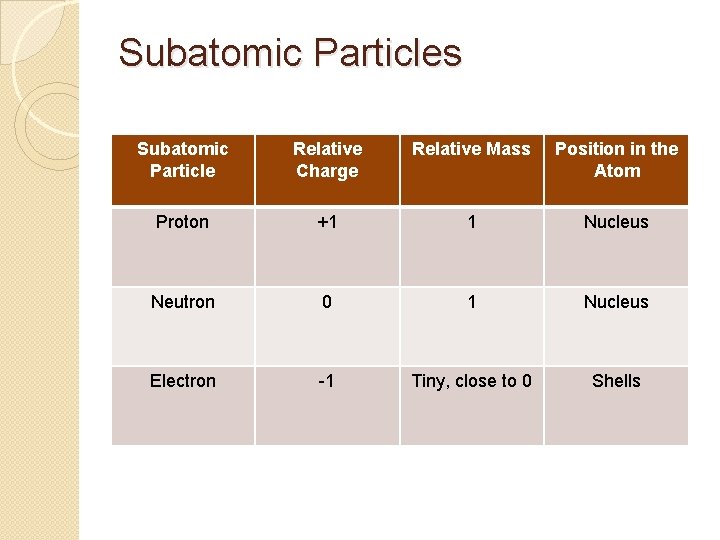
Subatomic Particles Subatomic Particle Relative Charge Relative Mass Position in the Atom Proton +1 1 Nucleus Neutron 0 1 Nucleus Electron -1 Tiny, close to 0 Shells

The Periodic Table �Look at your periodic tables and draw out an atom of: ◦ Lithium ◦ Sodium ◦ Fluorine �What do Li and Na have in common? �What do F and Li have in common? �Does this have anything to do with their placement on the periodic table?

Plenary �Write down one thing about atoms you are sure of, one thing you are unsure of and one thing you need to know more about.
- Slides: 15