What are the products of this doublereplacement chemical

What are the product(s) of this double-replacement chemical reaction? Fe. Cl 3 + 3 NH 4 OH →

Identify the type of reaction shown by this chemical equation: 2 Al + 6 HCl → 2 Al. Cl 3 + 3 H 2

Which type of chemical reaction would this be classified as? C 3 H 8 + 5 O 2 → 3 CO 2 + 4 H 2 O

Which type of reaction takes place in the presence of oxygen and produces carbon dioxide and water?

Use the activity series to predict which reaction will occur. a. Na. Br + I 2 b. KBr + F 2 c. Li. F + Cl 2 d. Na. Cl + I 2

How many total atoms are in 3 Na 2 SO 4?

In a chemical equation, (aq) after one of the substances means what?

Which type of reaction is 4 Al + 3 O 2 → 2 Al 2 O 3?

The equation below shows an example of which type of chemical reaction? NH 4 NO 3(s) N 2 O(g) + 2 H 2 O

Which is NOT a sign that a double displacement reaction has occurred? a. gas may be formed b. water may be produced c. a precipitate is formed d. the coefficients are equal

Identify the type of chemical reaction demonstrated by this equation: 2 KCl. O 3 → 2 KCl + 3 O 2
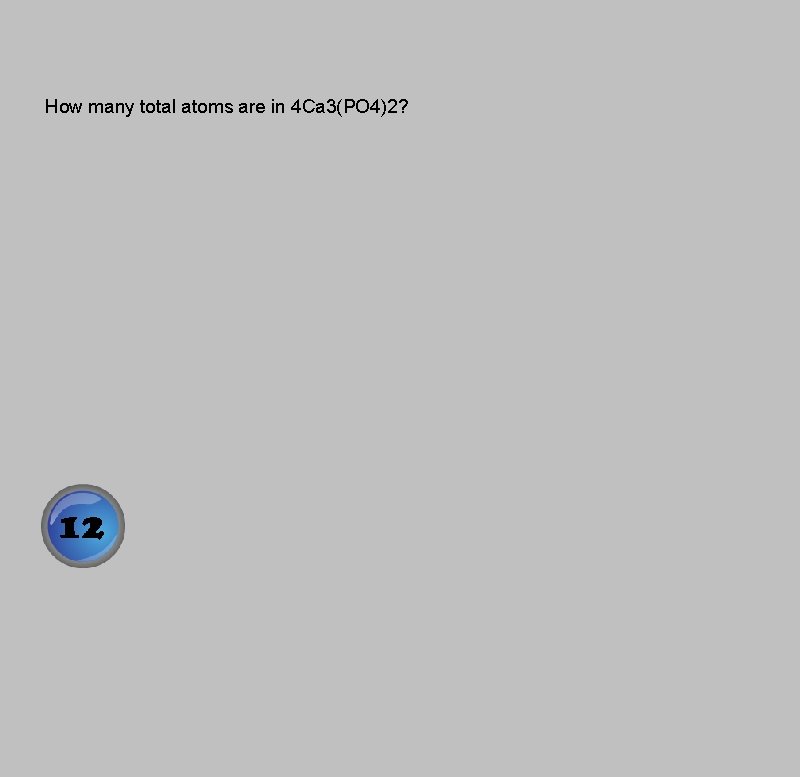
How many total atoms are in 4 Ca 3(PO 4)2?

Which are the spectator ions in the reaction shown? Ag. NO 3 (aq) + Na. Cl (aq) Na. NO 3 (aq) + Ag. Cl (s)

Use the activity series to determine if this reaction will take place. If the reaction will take place, complete the equation and balance it. Ca. Cl 2 + Mg

Write a balanced chemical equation for the following double-replacement reaction. zinc II sulfide reacts with hydrochloric acid to form zinc II chloride and hydrosulfuric acid

Write a balanced chemical equation for the following reaction. Fe 2 S 3 + O 2 Fe 2 O 3 + SO 2

Complete the equation and balance it. Aqueous strontium iodide plus aqueous potassium nitrate yields. . . ?

Write the complete ionic and net ionic equations. H 2 SO 4 (aq) + KOH (aq) K 2 SO 4 (aq) + H 2 O (l)

Balance the following combustion reaction. H 2 + O 2 H 2 O

What are the 3 things that are evidence of a double-replacement reaction?
- Slides: 21