What are the precursor compounds for secondary organic














- Slides: 51

What are the precursor compounds for secondary organic aerosols? What are the types of vegetation, vehicle exhaust, and burning that emit these precursors and under what conditions? R. Kamens, M. Jang, S. Lee, M. Jaoui, Depart. of Environ. Sci. and Eng. UNC-Chapel Hill kamens@unc. edu

Secondary organic aerosol (SOA) material may be defined as organic compounds that reside in the aerosol phase as a function of atmospheric reactions that occur in either the gas or particle phases.

The relative importance of precursors to secondary aerosol formation will depend on: 1. overall aerosol potential 2. atmospheric emissions 3. presence of other initiating reactants (O 3, OH, NO 3, sunlight, acid catalysts)

1. Terpenoid 2. Aromatic 3. Particle Phase Reactions (aldehydes and alcohols)

Leonardo Da Vinvi described blue haze and thought that plant emissions were its source…(Went, 1959) Da Vinvi believed that it was due to water moisture emitted from the plants

F. W. Went published papers on biogenic emissions from vegetation over 40 years ago. He posed the question, “what happens to 17. 5 x 107 tons of terpene-like hydrocarbons or slightly oxygenated hydrocarbons once they are in the atmosphere? ” Went suggested that terpenes are removed from the atmosphere by reaction with ozone and demonstrated “blue haze” formation by adding crushed pine or fir needles to a jar with dilute ozone.

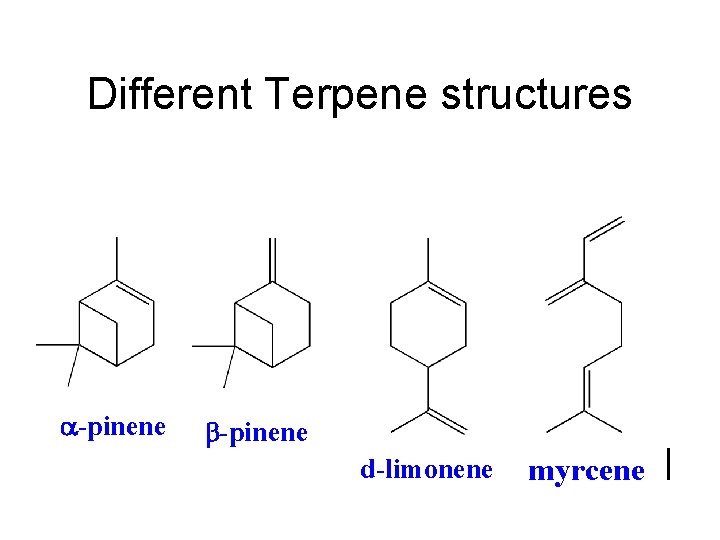
Different Terpene structures a-pinene b-pinene d-limonene myrcene

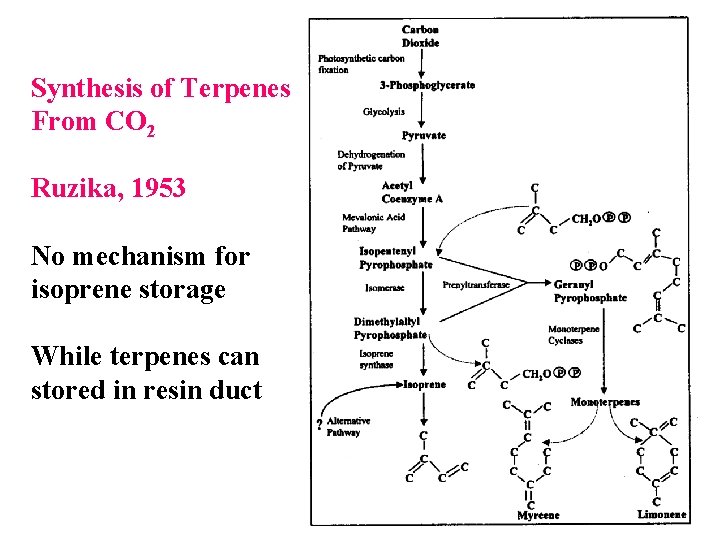
Synthesis of Terpenes From CO 2 Ruzika, 1953 No mechanism for isoprene storage While terpenes can stored in resin duct

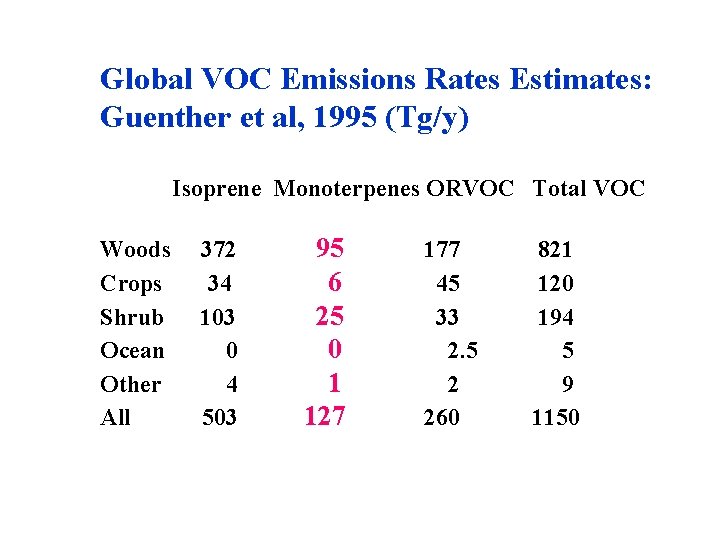
Global VOC Emissions Rates Estimates: Guenther et al, 1995 (Tg/y) Isoprene Monoterpenes ORVOC Total VOC Woods 372 95 Crops 34 6 Shrub 103 25 Ocean 0 0 Other 4 1 All 503 127 177 45 33 2. 5 2 260 821 120 194 5 9 1150

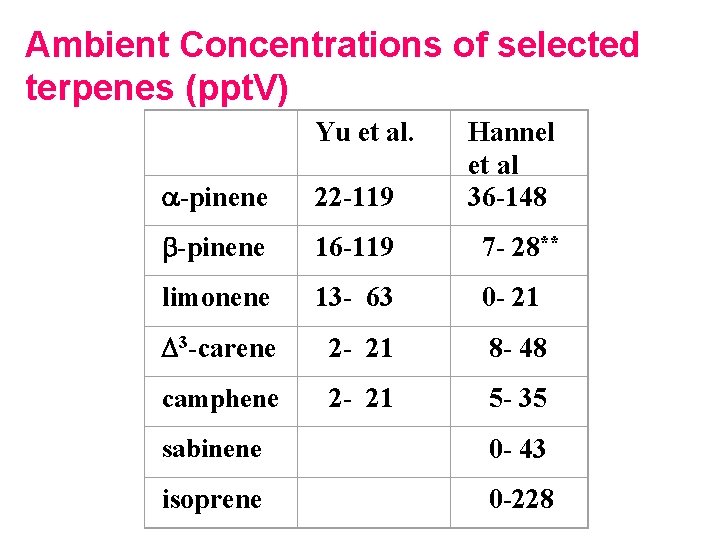
Ambient Concentrations of selected terpenes (ppt. V) Yu et al. a-pinene 22 -119 Hannel et al 36 -148 b-pinene 16 -119 7 - 28** limonene 13 - 63 0 - 21 D 3 -carene 2 - 21 8 - 48 camphene 2 - 21 5 - 35 sabinene 0 - 43 isoprene 0 -228

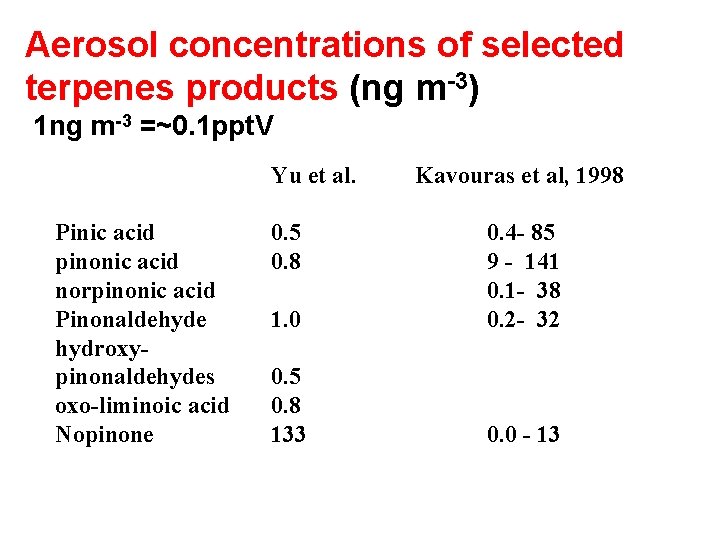
Aerosol concentrations of selected terpenes products (ng m-3) 1 ng m-3 =~0. 1 ppt. V Yu et al. Pinic acid pinonic acid norpinonic acid Pinonaldehyde hydroxypinonaldehydes oxo-liminoic acid Nopinone 0. 5 0. 8 Kavouras et al, 1998 1. 0 0. 4 - 85 9 - 141 0. 1 - 38 0. 2 - 32 0. 5 0. 8 133 0. 0 - 13

Mechanisms can often explain the formation of products

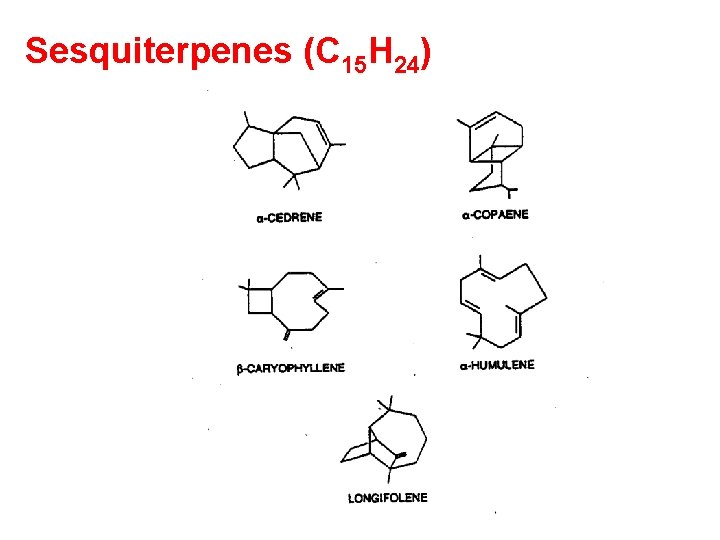
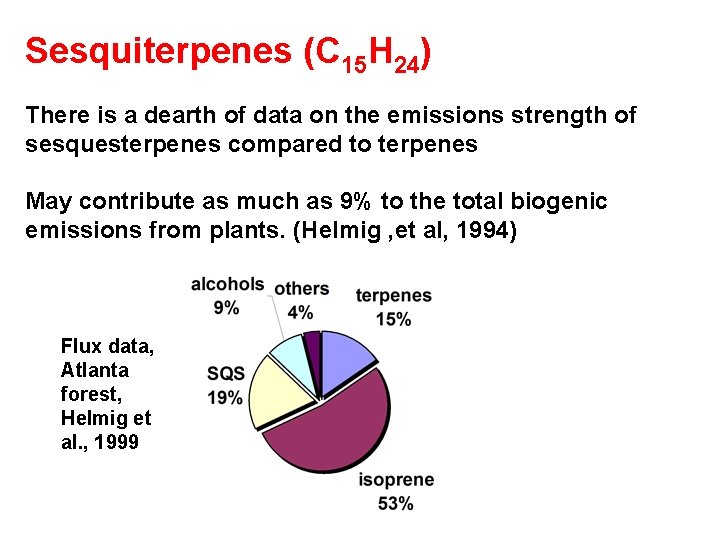
Sesquiterpenes (C 15 H 24)

Sesquiterpenes (C 15 H 24) There is a dearth of data on the emissions strength of sesquesterpenes compared to terpenes May contribute as much as 9% to the total biogenic emissions from plants. (Helmig , et al, 1994) Flux data, Atlanta forest, Helmig et al. , 1999

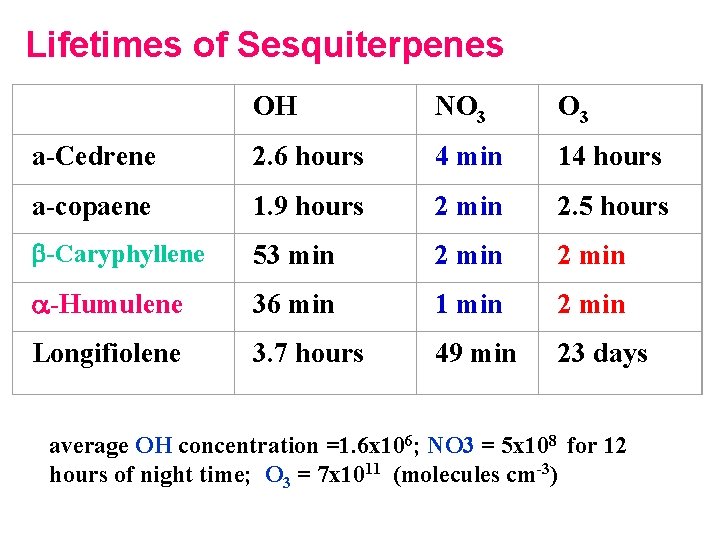
Lifetimes of Sesquiterpenes OH NO 3 a-Cedrene 2. 6 hours 4 min 14 hours a-copaene 1. 9 hours 2 min 2. 5 hours b-Caryphyllene 53 min 2 min a-Humulene 36 min 1 min 2 min Longifiolene 3. 7 hours 49 min 23 days average OH concentration =1. 6 x 106; NO 3 = 5 x 108 for 12 hours of night time; O 3 = 7 x 1011 (molecules cm-3)

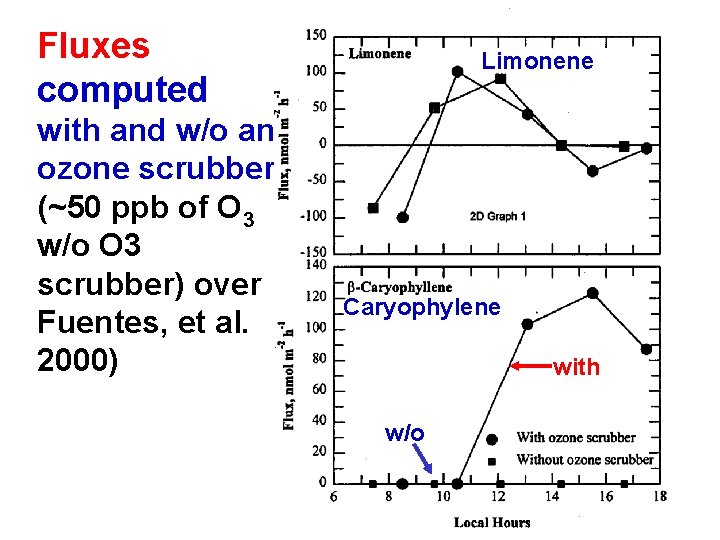
Fluxes computed with and w/o an ozone scrubber (~50 ppb of O 3 w/o O 3 scrubber) over Fuentes, et al. 2000) Limonene Caryophylene with w/o

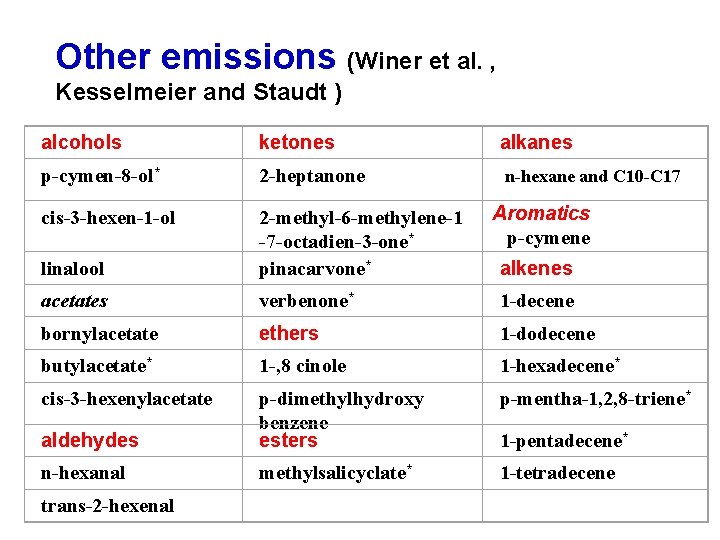
Other emissions (Winer et al. , Kesselmeier and Staudt ) alcohols ketones alkanes p-cymen-8 -ol* 2 -heptanone n-hexane and C 10 -C 17 cis-3 -hexen-1 -ol linalool 2 -methyl-6 -methylene-1 -7 -octadien-3 -one* pinacarvone* acetates verbenone* 1 -decene bornylacetate ethers 1 -dodecene butylacetate* 1 -, 8 cinole 1 -hexadecene* cis-3 -hexenylacetate p-mentha-1, 2, 8 -triene* aldehydes p-dimethylhydroxy benzene esters n-hexanal methylsalicyclate* 1 -tetradecene trans-2 -hexenal Aromatics p-cymene alkenes 1 -pentadecene*

Factors that influence emissions 1. Temperature 2. light 3. injury

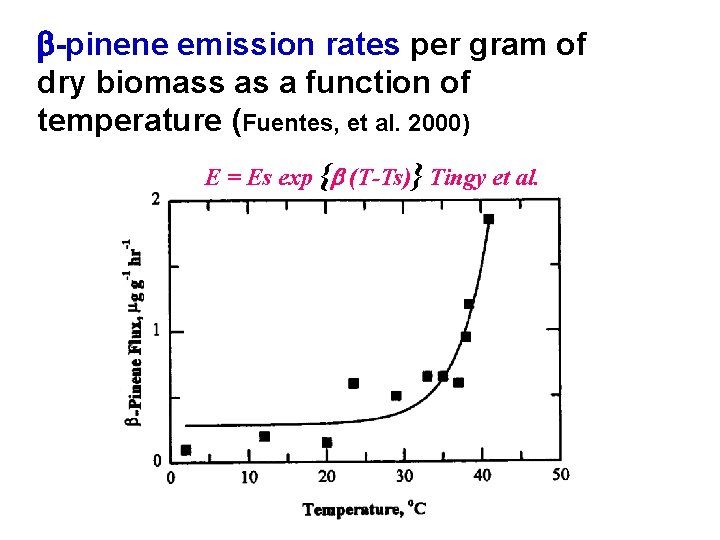
b-pinene emission rates per gram of dry biomass as a function of temperature (Fuentes, et al. 2000) E = Es exp {b (T-Ts)} Tingy et al.

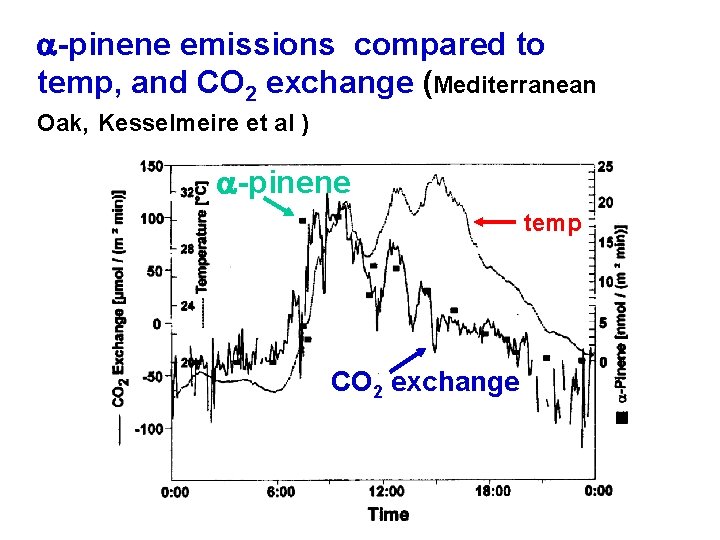
a-pinene emissions compared to temp, and CO 2 exchange (Mediterranean Oak, Kesselmeire et al ) a-pinene temp CO 2 exchange

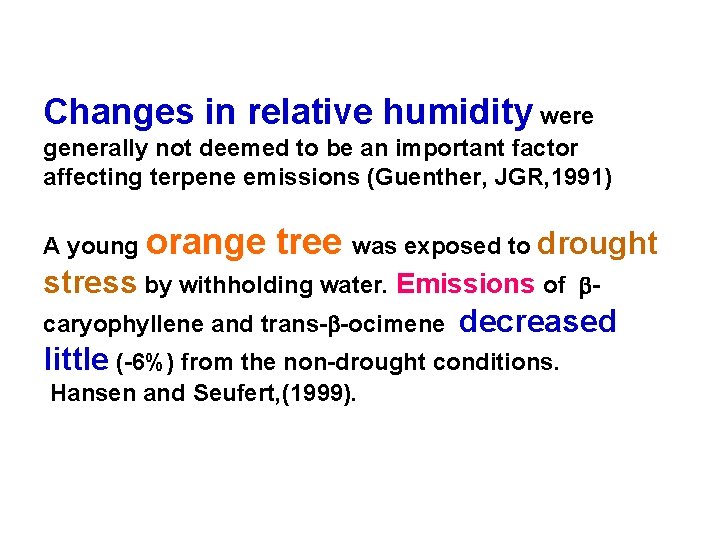
Changes in relative humidity were generally not deemed to be an important factor affecting terpene emissions (Guenther, JGR, 1991) A young orange tree was exposed to drought stress by withholding water. Emissions of bcaryophyllene and trans-b-ocimene decreased little (-6%) from the non-drought conditions. Hansen and Seufert, (1999).

Emissions from drought-stressed apple leaves seem to show significant increases in hexanal, 2 hexenal, and hexanol (Ebel et al. 1995) Shade, et al (G. Res. Let. , 1999) measured increases in monoterpene emissions of D-3 carene over a ponderosa pine plantation in the Sierra Nevada mountains after rain events and under high humidity, Tingey equation is corrected by multiplying by a relative humidity factor, BET= cx. RHn)/((1 -c. RHn)x(1+(c-1)x. RHn) where c a constant, and RHn a normalized relative humidity = (%relative humididy-18)/82

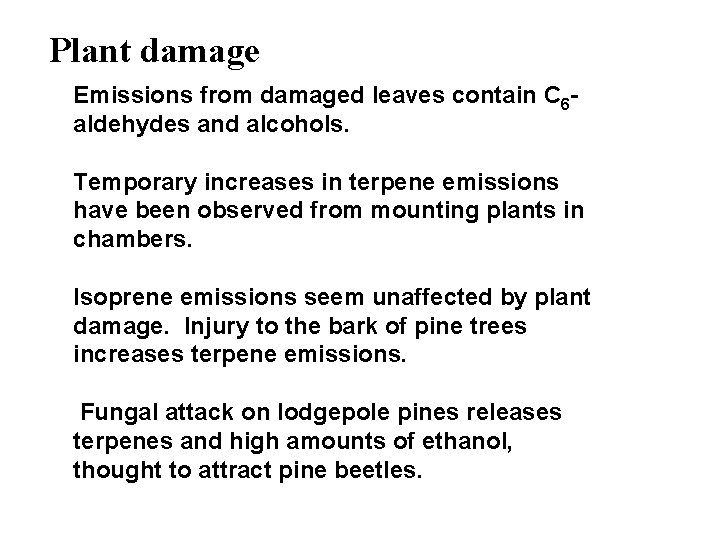
Plant damage Emissions from damaged leaves contain C 6 aldehydes and alcohols. Temporary increases in terpene emissions have been observed from mounting plants in chambers. Isoprene emissions seem unaffected by plant damage. Injury to the bark of pine trees increases terpene emissions. Fungal attack on lodgepole pines releases terpenes and high amounts of ethanol, thought to attract pine beetles.

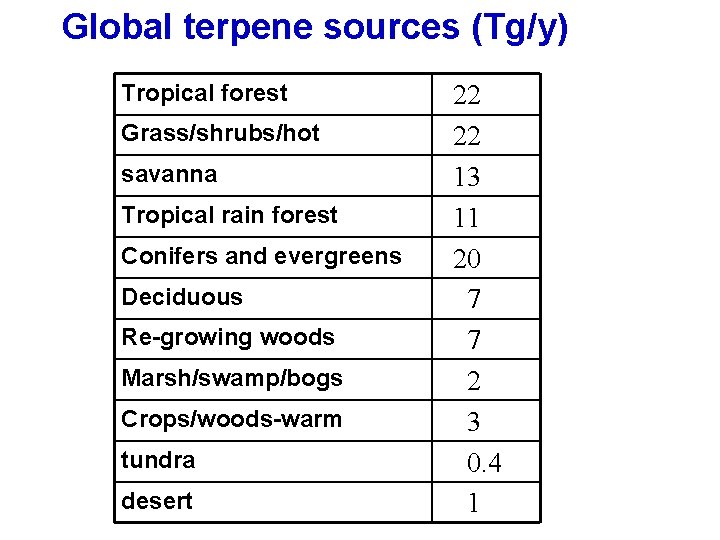
Global terpene sources (Tg/y) Tropical forest Grass/shrubs/hot savanna Tropical rain forest Conifers and evergreens Deciduous Re-growing woods Marsh/swamp/bogs Crops/woods-warm tundra desert 22 13 11 20 7 7 2 3 0. 4 1

Aerosol formation from Terpenes

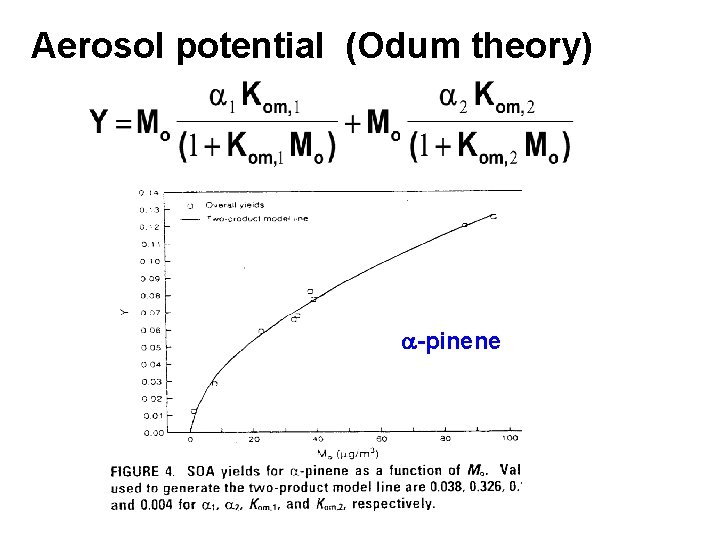
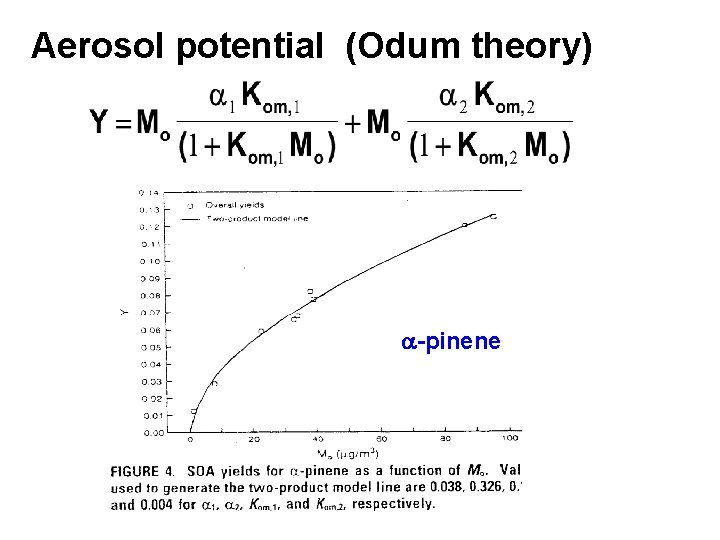
Aerosol potential (Odum theory) a-pinene


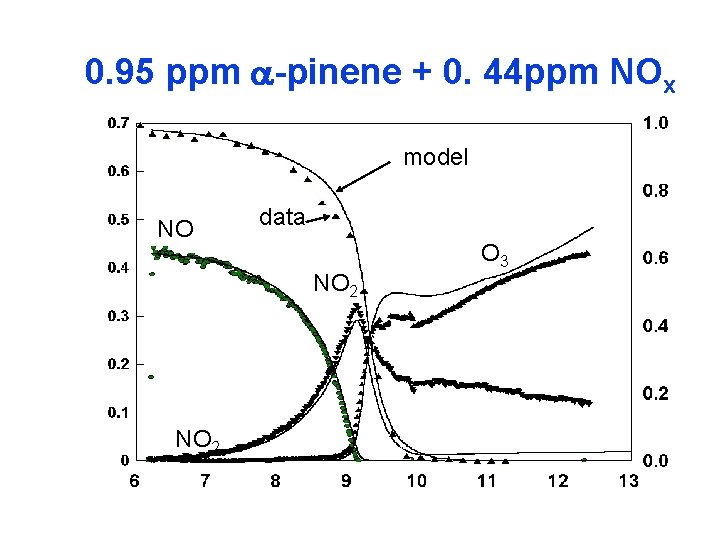
0. 95 ppm a-pinene + 0. 44 ppm NOx model NO data NO 2 O 3

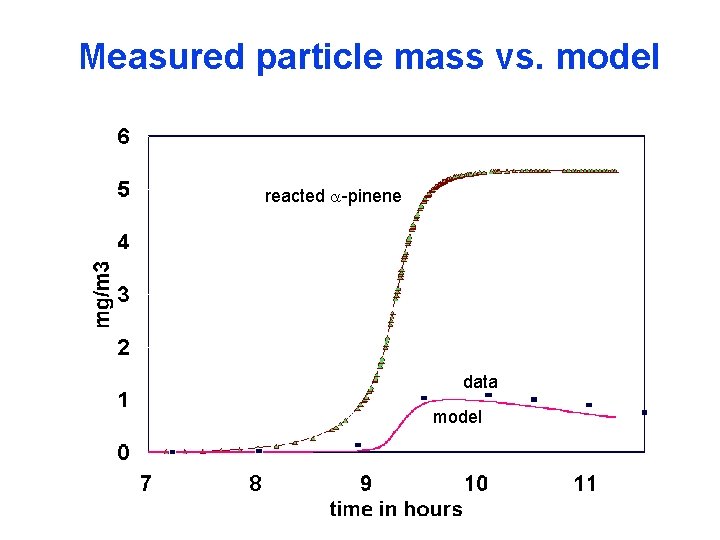
Measured particle mass vs. model reacted a-pinene data model

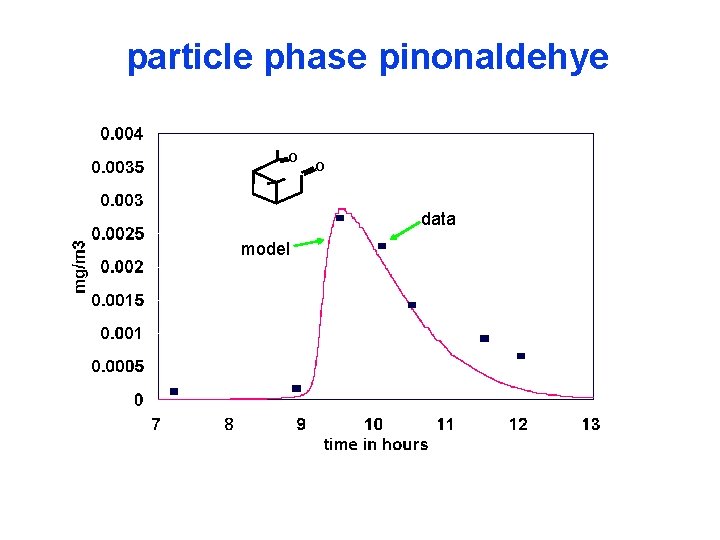
particle phase pinonaldehye O O data model

Aerosol potential (Odum theory) a-pinene

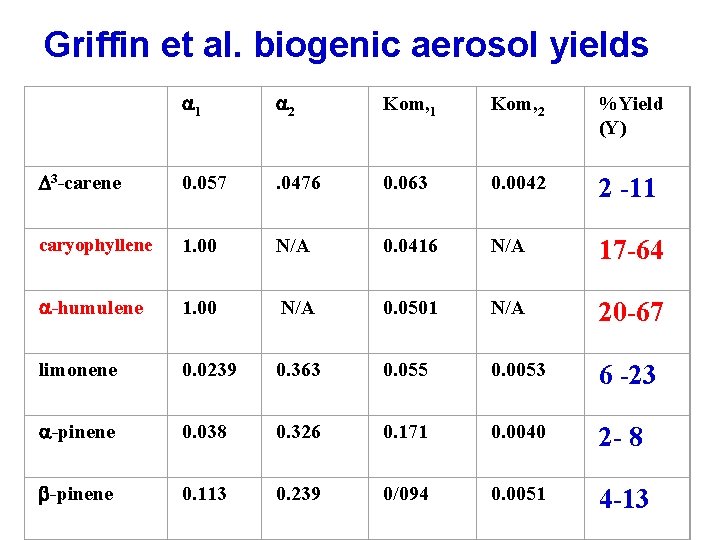
Griffin et al. biogenic aerosol yields a 1 a 2 Kom, 1 Kom, 2 %Yield (Y) D 3 -carene 0. 057 . 0476 0. 063 0. 0042 2 -11 caryophyllene 1. 00 N/A 0. 0416 N/A 17 -64 a-humulene 1. 00 N/A 0. 0501 N/A 20 -67 limonene 0. 0239 0. 363 0. 055 0. 0053 6 -23 a-pinene 0. 038 0. 326 0. 171 0. 0040 2 - 8 b-pinene 0. 113 0. 239 0/094 0. 0051 4 -13

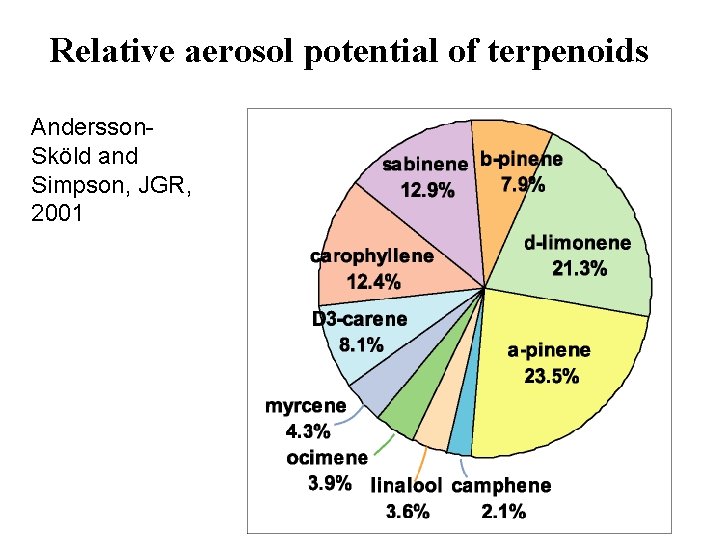
Relative aerosol potential of terpenoids Andersson. Sköld and Simpson, JGR, 2001

Griffin et al, JGR, 2000 Used a global photochemcial model to estimate the amount of terpenes and other biogenics that are reacted, DROGi. These were used in conjunction with specific compound “Odum fitting” constants to estimate total boigenic aerosol production on a yearly basis. This may be a conservative estimate because the fitting contents are derived at 308 K, does not consider other aerosol surfaces, or particle phase reactions

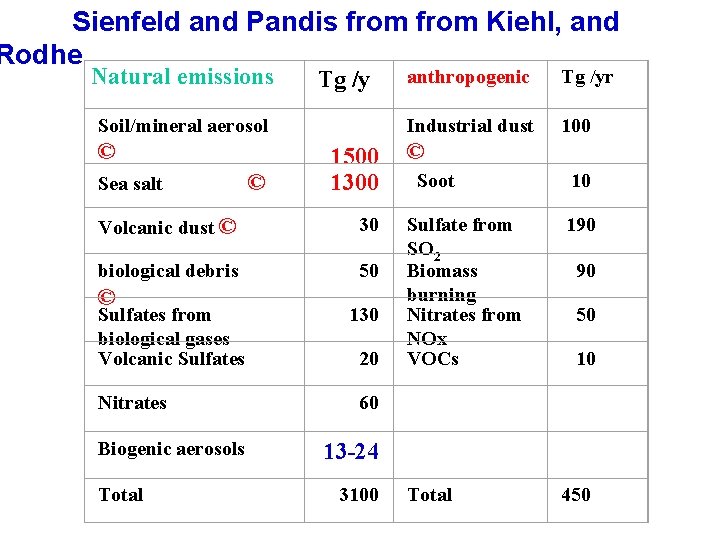
Sienfeld and Pandis from Kiehl, and Rodhe Natural emissions anthropogenic Tg /y Soil/mineral aerosol © Sea salt © Tg /yr Industrial dust 100 1500 1300 Volcanic dust © 30 biological debris 50 © Soot 10 Sulfates from 130 biological gases Volcanic Sulfates 20 Sulfate from 190 SO 2 Biomass 90 burning Nitrates from 50 NOx VOCs 10 Nitrates 60 Biogenic aerosols 13 -24 Total 450 © Total 3100

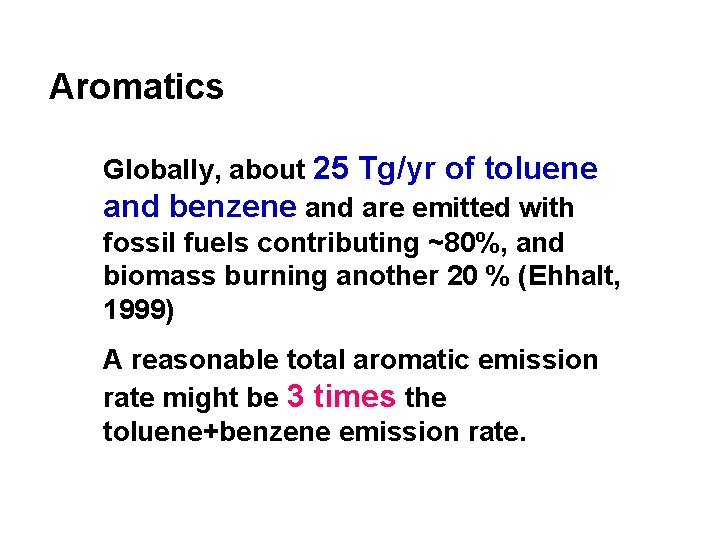
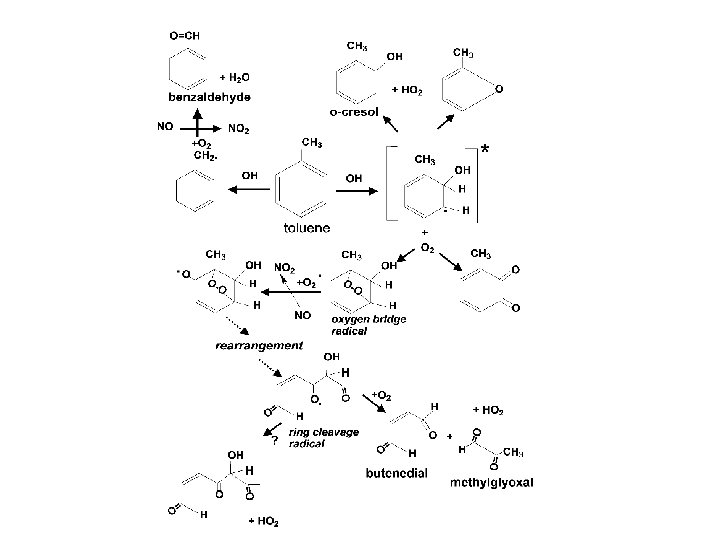
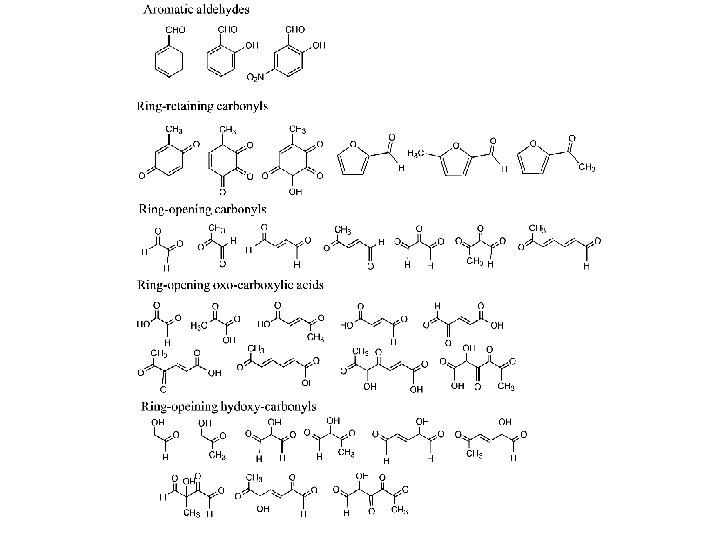
Aromatics Globally, about 25 Tg/yr of toluene and benzene and are emitted with fossil fuels contributing ~80%, and biomass burning another 20 % (Ehhalt, 1999) A reasonable total aromatic emission rate might be 3 times the toluene+benzene emission rate.

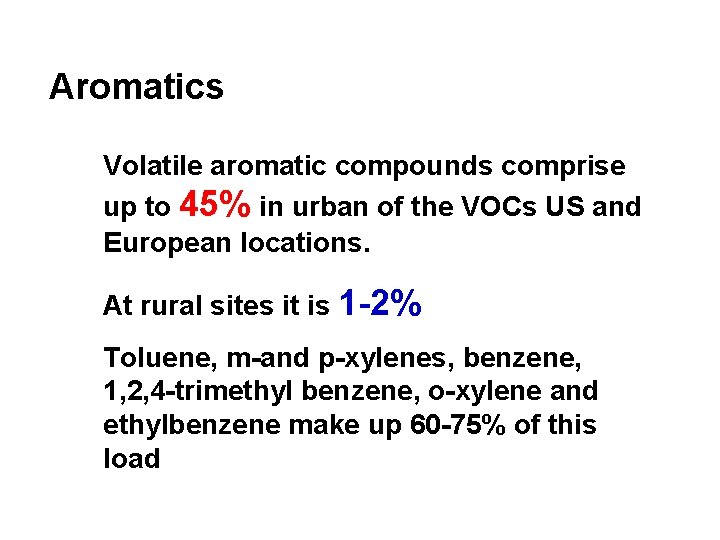
Aromatics Volatile aromatic compounds comprise up to 45% in urban of the VOCs US and European locations. At rural sites it is 1 -2% Toluene, m-and p-xylenes, benzene, 1, 2, 4 -trimethyl benzene, o-xylene and ethylbenzene make up 60 -75% of this load

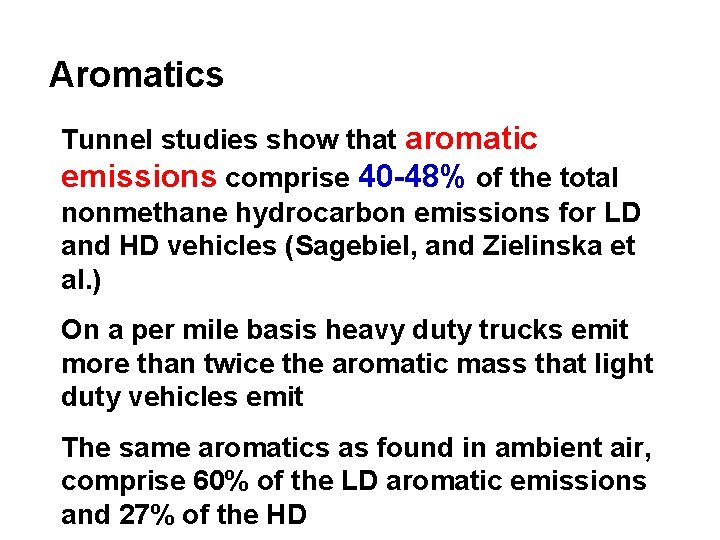
Aromatics Tunnel studies show that aromatic emissions comprise 40 -48% of the total nonmethane hydrocarbon emissions for LD and HD vehicles (Sagebiel, and Zielinska et al. ) On a per mile basis heavy duty trucks emit more than twice the aromatic mass that light duty vehicles emit The same aromatics as found in ambient air, comprise 60% of the LD aromatic emissions and 27% of the HD

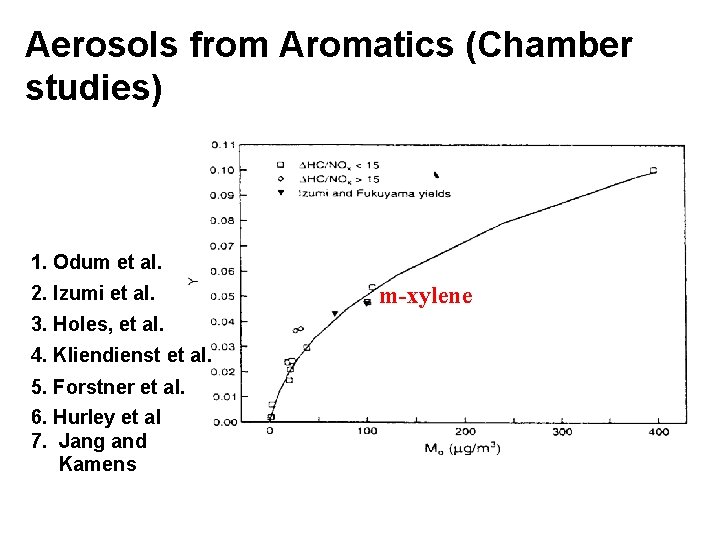
Aerosols from Aromatics (Chamber studies) 1. Odum et al. 2. Izumi et al. 3. Holes, et al. 4. Kliendienst et al. 5. Forstner et al. 6. Hurley et al 7. Jang and Kamens m-xylene



Particle phase reactions In UNC chamber experiments partitioning “Pankow” coefficients for aldehydes are much higher than predicted partitioning coefficients, calculated from the vapor pressures and activity coefficients (Jang and Kamens, ES&T, 2001, Kamens and Jaoui, ES&T, 2001 )

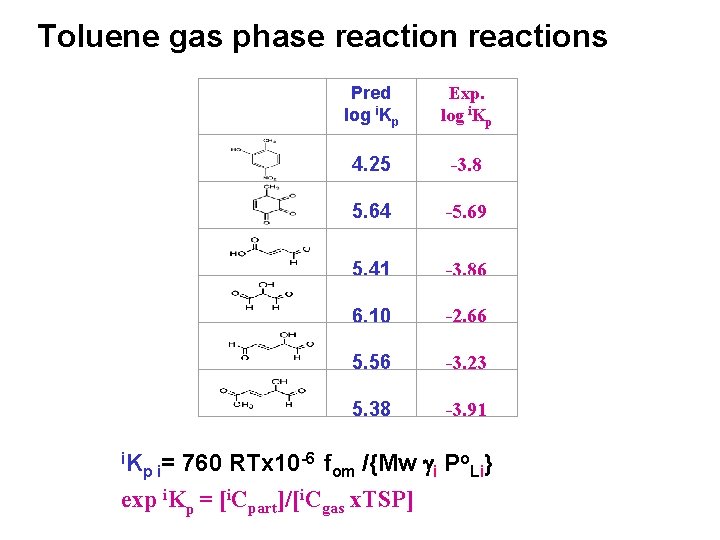
Toluene gas phase reactions i. K Pred log i. Kp Exp. log i. Kp 4. 25 -3. 8 5. 64 -5. 69 5. 41 6. 10 5. 56 5. 38 -3. 86 -2. 66 -3. 23 -3. 91 -6 f o } = 760 RTx 10 /{Mw g P p i om i Li exp i. Kp = [i. Cpart]/[i. Cgas x. TSP]

Particle phase reactions Ziemann and Tobias have reported the formation of hemiacetals in the particle phase of secondary organic aerosols

• Aldehyde functional groups can react in the aerosol phase through heterogeneous reactions via hydration, polymerization, and hemiacetal/acetal formation with alcohols. • Aldehyde reactions can be radically accelerated by acid catalysts such as particle sulfuric acid (Jang and Kamens, ES&T, 2001)

Why don’t we see these large highly oxygenated compounds? ? Reverse reactions to the original aldehyde parent structures can occur during sample work up/solvent extraction procedures;

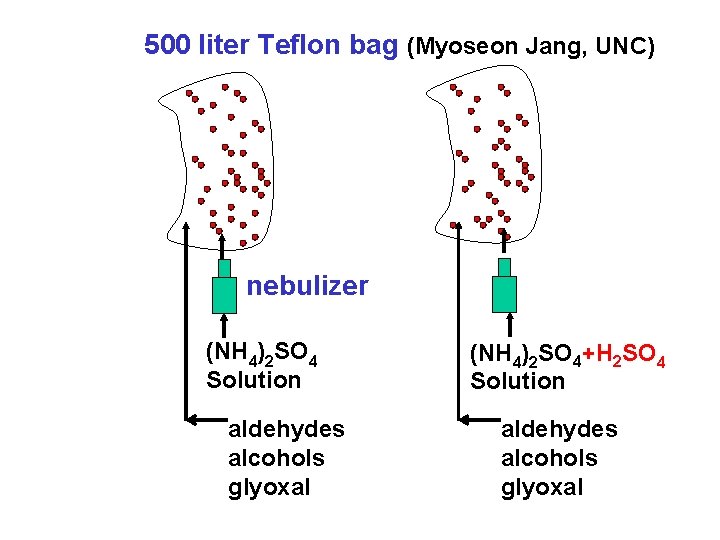
500 liter Teflon bag (Myoseon Jang, UNC) nebulizer (NH 4)2 SO 4 Solution (NH 4)2 SO 4+H 2 SO 4 Solution aldehydes alcohols glyoxal

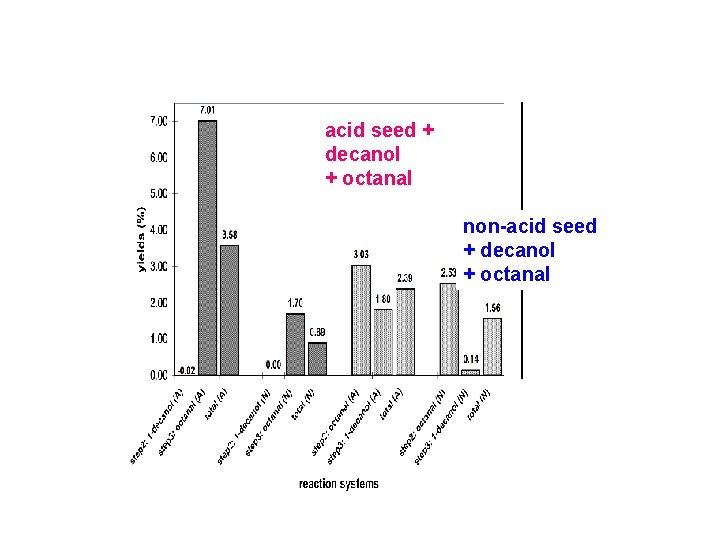
acid seed + decanol + octanal non-acid seed + decanol + octanal

• To demonstrate the acid catalyzed aldehyde reaction, octanal was reacted directly on a Zn. Se FTIR window by adding small amounts of aqueous H 2 SO 4 acid catalyst solution (0. 005 M). The spectra of the octanal/acid-catalyst system changed progressively as a function of time • The aldehydic C-H stretching at 2715 cm-1 immediately disappeared, the C=O stretching band at 1726 cm-1 gradually decreased • and the OH stretching at 3100 -3600 cm-1 increased as hydrates formed.

Future research areas. §Determine the importance of particle phase reactions as a source of SOA. §Determine the importance of sesquiterpenes in SOA formation. §Clarification of the impact of drought and relative humidity on biogenic emissions is needed so these factors can be incorporated into emission models.

Future research areas (cont. ) §Integrated chemical mechanisms for predicting SOA from biogenics and aromatic precursors. §New analytical techniques to detect and quantify particle phase reactions. These need to be non-invasive or “chemically soft” so that complex particle phase reactions products are not decomposed.