What are the physical properties of matter Physical






















- Slides: 41

What are the physical properties of matter?

Physical Properties

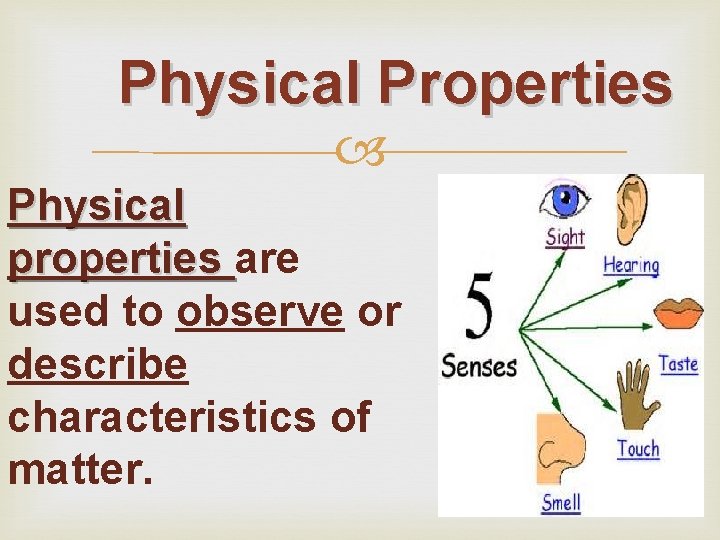
Physical Properties Physical properties are used to observe or describe characteristics of matter.

Physical Properties • How does it look? … Red? Blue? Shiny? Reflective? Dull? • What is it made of? … wood? Glass? • How does it feel? … Pointy? Soft? Fuzzy? • How does it smell? … Sweet? Rotten? Minty?

Physical Properties Physical properties DO NOT change the structure of the matter when it is observed or described. Structure refers to how the atoms are put together.

Physical Properties that can be observed or measured without changing the chemical nature of the matter: • color • size • shape • smell • mass ou sta r wil ndar on l foc d u ph thes s pro ysica e pe l rtie s • volume • density • solubility • boiling point • melting point • freezing point

Physical Properties: Volume

Physical Properties: Volume is the amount of space that an object takes up.

Physical Properties: Volume is the amount of space that an object takes up. All matter has mass and volume!

Physical Properties: Density

Physical Properties: If you pick up a Density baseball and a tennis ball, which one would feel heavier? Even though they are both of similar size, the baseball will feel heavier because it is denser.

Physical Properties: Density is how we measure how much matter is in a given amount of space. t a h w t s i u h B t s e o d ly l a e How packed r ? n a e together the m matter is in an object

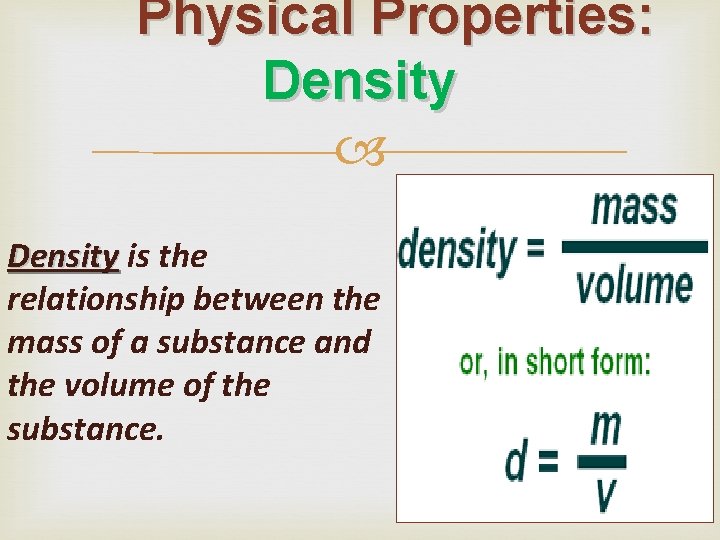
Physical Properties: Density is the relationship between the mass of a substance and the volume of the substance.

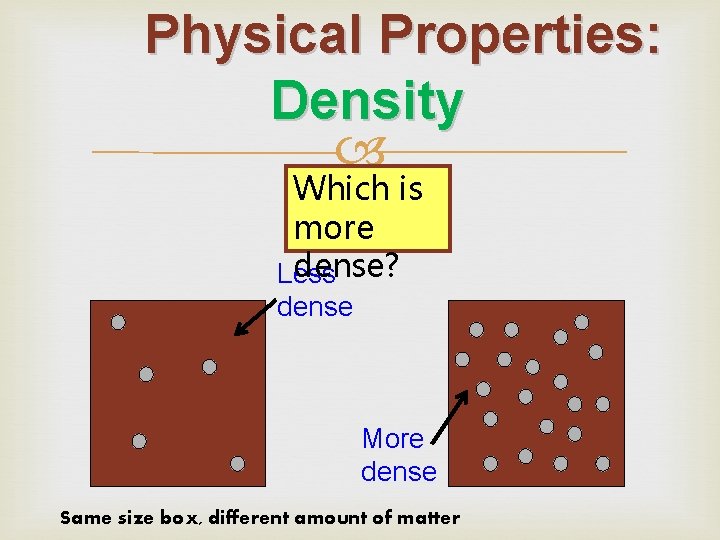
Physical Properties: Density Which is more dense? Less dense More dense Same size box, different amount of matter

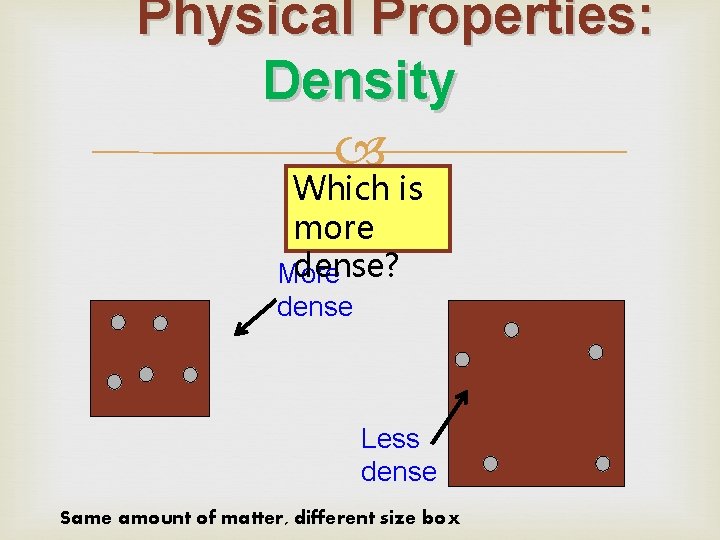
Physical Properties: Density Which is more dense? More dense Less dense Same amount of matter, different size box

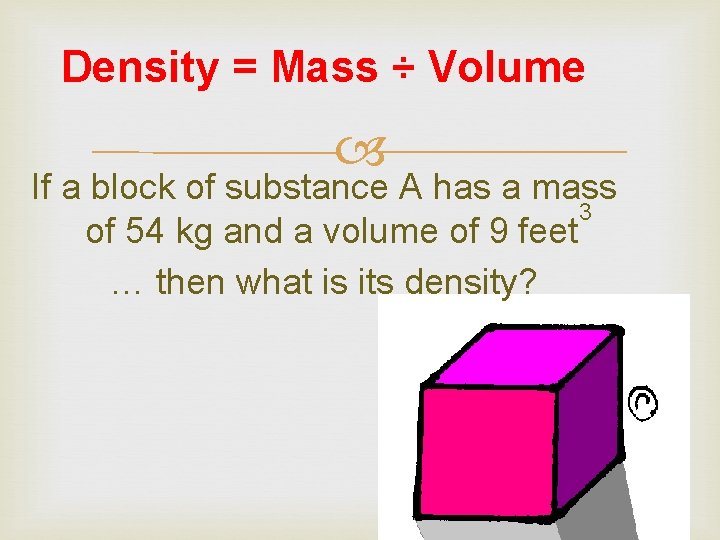
Density = Mass ÷ Volume If a block of substance A has a mass 3 of 54 kg and a volume of 9 feet … then what is its density?

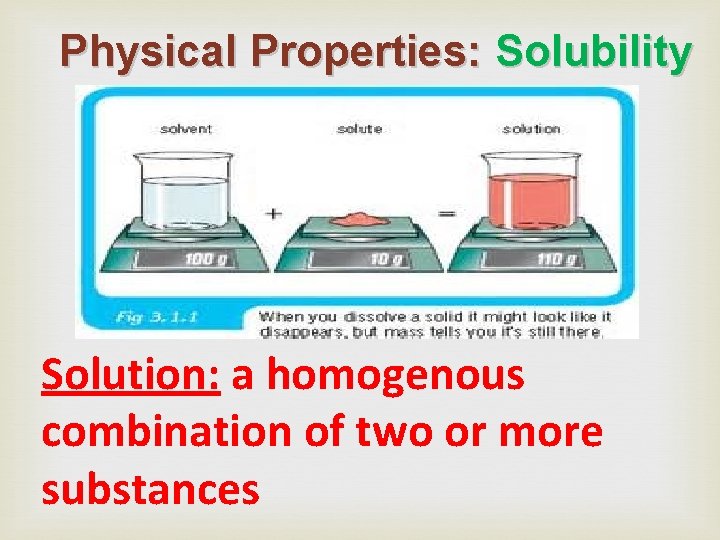
Physical Properties: Solubility

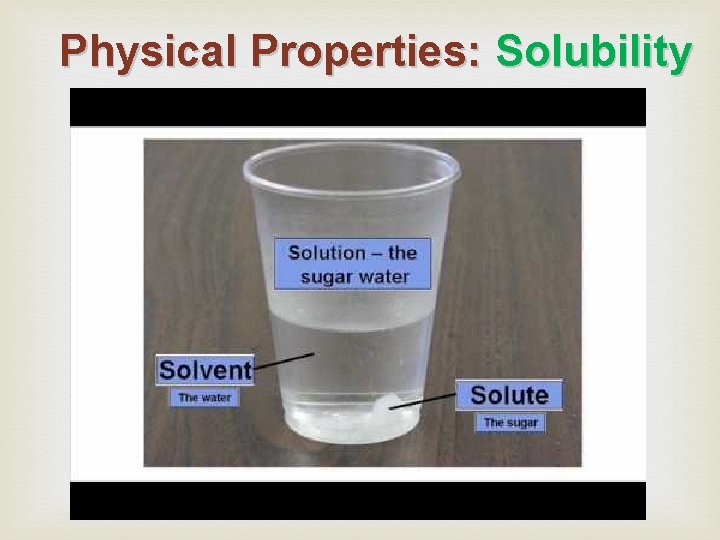
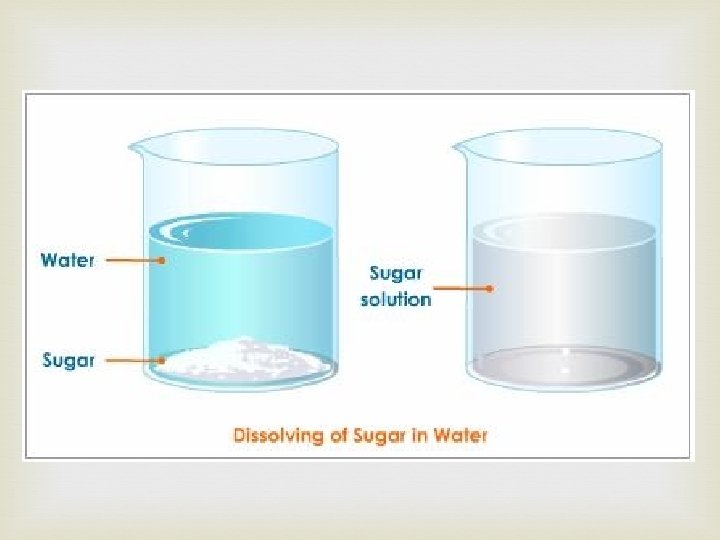
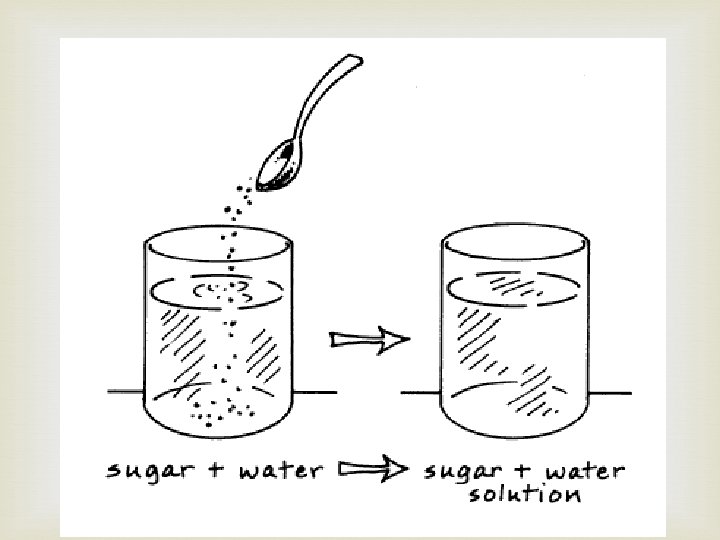
Physical Properties: Solubility means the amount of a substance that can be dissolved in another substance. How much sugar can dissolve in water? solute solvent (water is the most common solvent) *Temperature affects solubility

Physical Properties: Solubility Solute: the part of a solution that gets dissolved____ Solvent: the part of a solution that does the dissolving

Physical Properties: Solubility

Physical Properties: Solubility Solution: a homogenous combination of two or more substances



Hot Cocoa is a solution

Tomato Soup is a solution

Kool-Aid is a solution

A Solution is a mixture of 2 or more substances, where one in the other, substance dissolves and cannot be physically separated.

Physical Properties: Solubility Mixture: a heterogeneous combination of two or more substances that keep their properties

Theses are examples of MIXTURES

Chicken Noodle Soup is a Mixture

A Mixture is a combination of 2 or more substances that are not chemically united. Mixtures can be separated into different, pure particles.

Physical Properties: Boiling Point

Physical Properties temperature Boiling Point – the at which a substance turns from a liquid to a gas

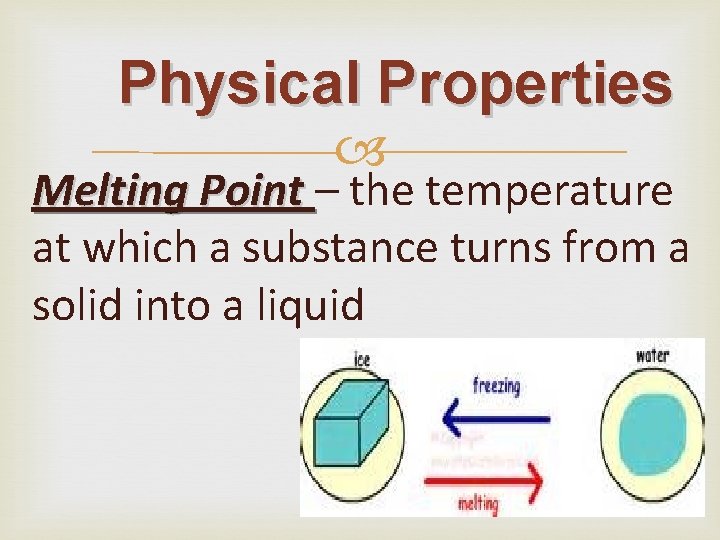
Physical Properties: Melting Point

Physical Properties Melting Point – the temperature at which a substance turns from a solid into a liquid

Physical Properties Melting Point When a solid turns into a liquid it is called melting. There is a temperature at which this happens called the melting point. As the energy in the molecules increases from a rise in temperature, the molecules start moving faster. Soon they have enough energy to break free of their rigid structure and start moving around more easily. The matter becomes a liquid. The melting point for water is 0 degrees C (32 degrees F).

Physical Properties: Freezing Point

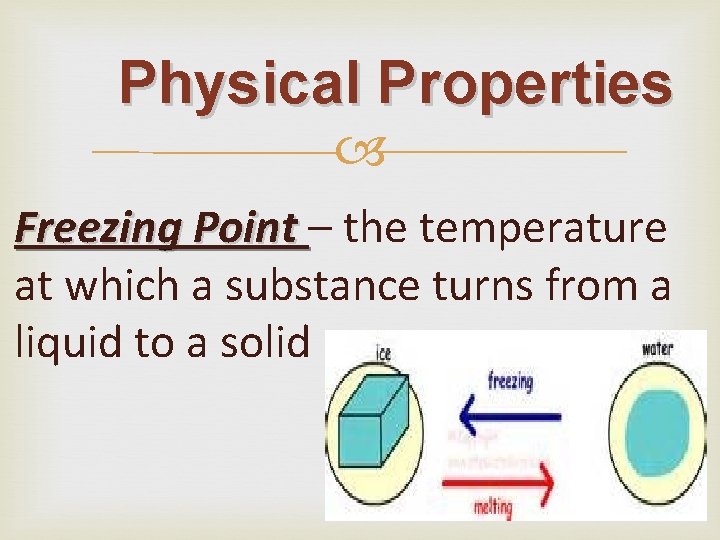
Physical Properties Freezing Point – the temperature at which a substance turns from a liquid to a solid

Physical Properties Freezing Point When a liquid turns into a solid it is called freezing. There is a temperature at which this happens called the freezing point. As the energy in the molecules decreases from a fall in temperature, the molecules start moving slower. Soon they don’t have enough energy to move easily and they are confined to a rigid structure. The matter becomes a solid. The freezing point for water is 0 degrees C (32 degrees F).

Physical Properties of a substances The temperature freezing point and the temperature of a substances melting point are the same temperature!

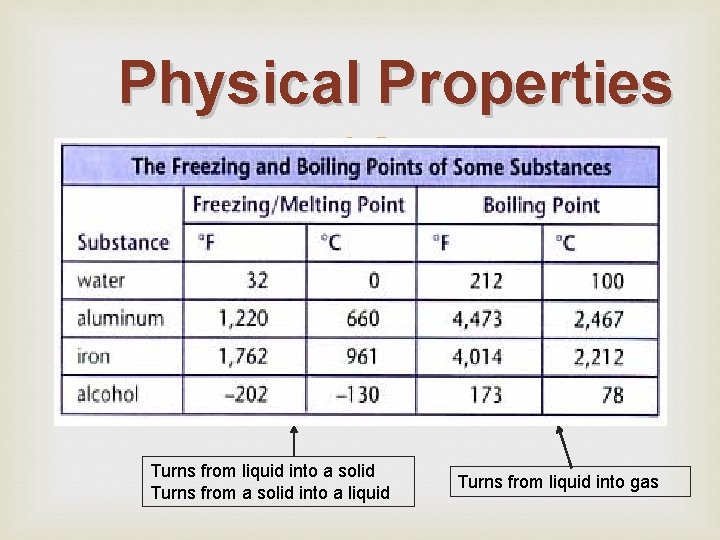
Physical Properties Turns from liquid into a solid Turns from a solid into a liquid Turns from liquid into gas