What are the different classifications of matter Solid




- Slides: 13

What are the different classifications of matter?

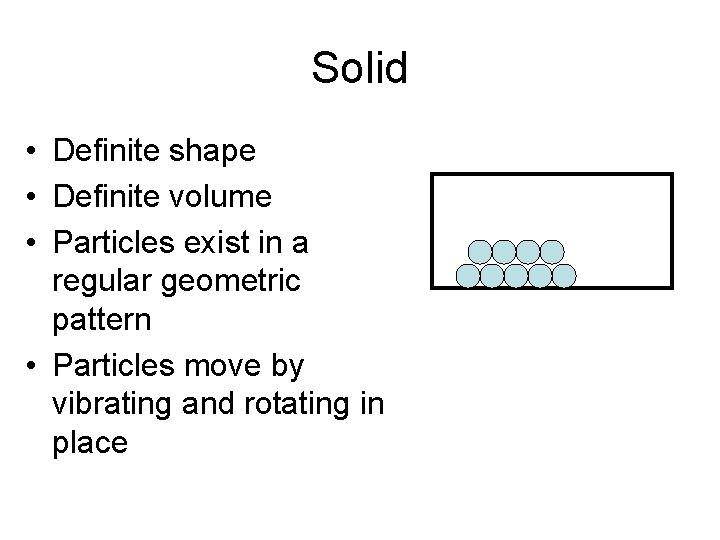
Solid • Definite shape • Definite volume • Particles exist in a regular geometric pattern • Particles move by vibrating and rotating in place

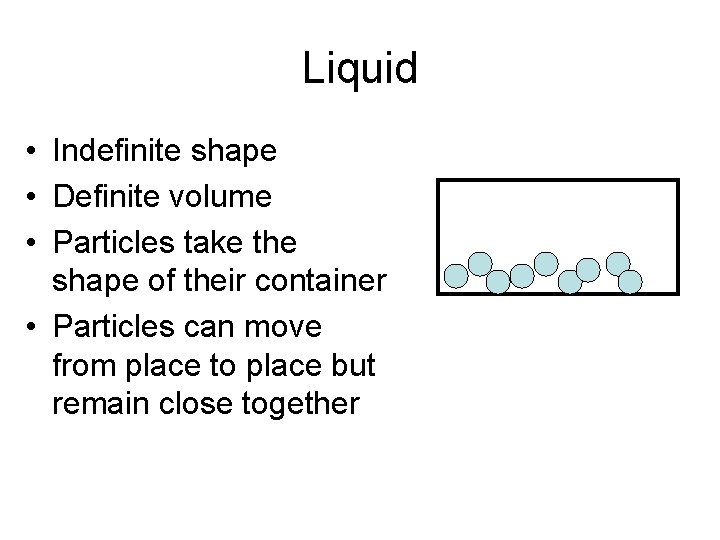
Liquid • Indefinite shape • Definite volume • Particles take the shape of their container • Particles can move from place to place but remain close together

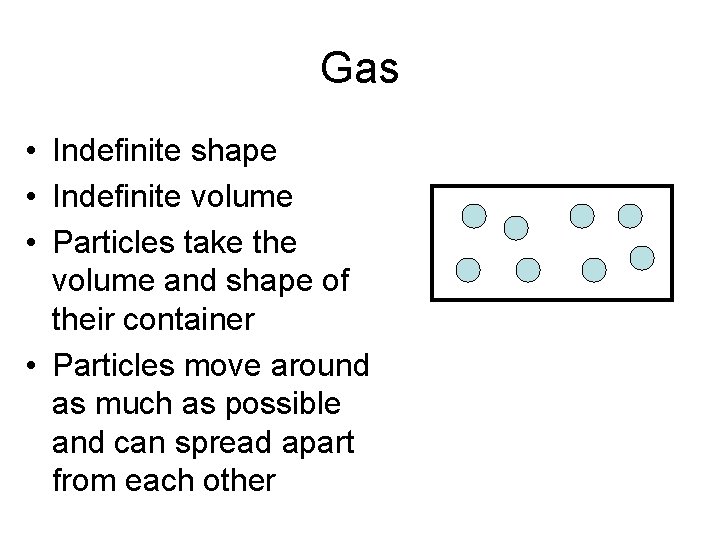
Gas • Indefinite shape • Indefinite volume • Particles take the volume and shape of their container • Particles move around as much as possible and can spread apart from each other

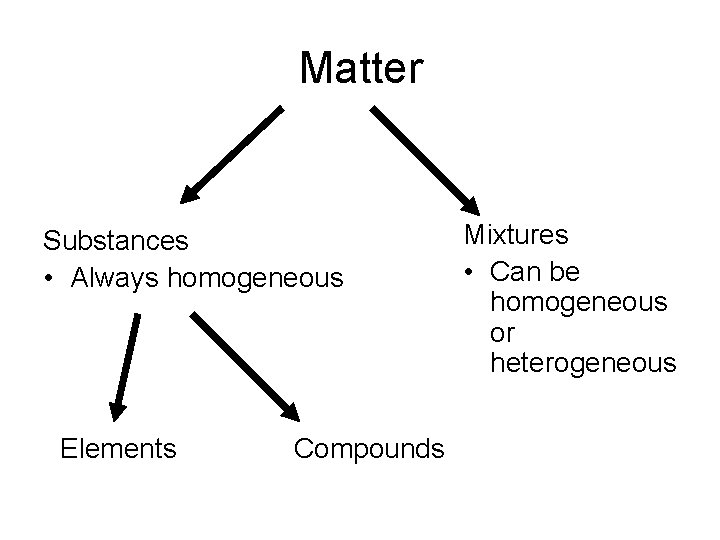
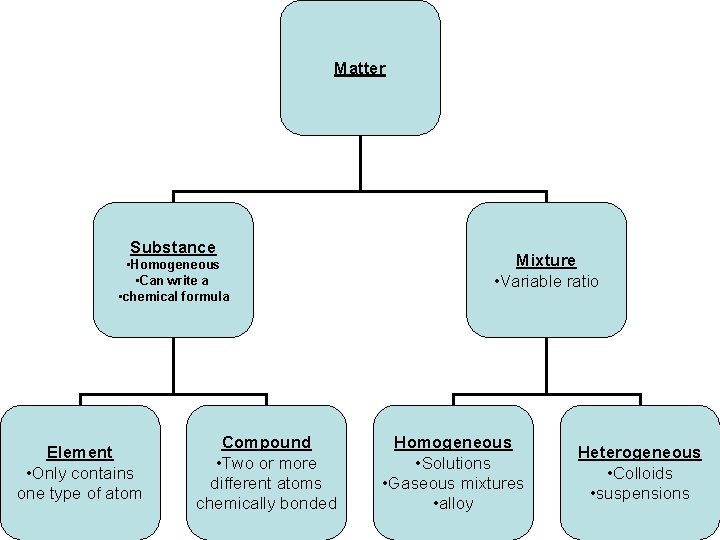
Matter Substances • Always homogeneous Elements Compounds Mixtures • Can be homogeneous or heterogeneous

Element • Only made up of one type of atom • Can be monatomic (ex: C, Al, K) • Can be diatomic (ex: O 2, N 2, Cl 2) – HOFBr. INCl • Can be polyatomic (sulfur can exist as S 8) • Always a homogeneous sample • Cannot be decomposed by physical or chemical change • Allotrope: Different forms of the same element at the same temp and pressure that have different structures and different physical and chemical properties – Example: Carbon can exist as graphite, diamond, and buckminsterfullerene at STP

Matter Substances • Always homogeneous Elements Compounds Mixtures • Can be homogeneous or heterogeneous

Compound • Made up of 2 or more different types of atoms chemically combined • Exists in a definite ratio – Carbon dioxide is CO 2. It always has a carbon: oxygen ratio of 1: 2. • The compound has different chemical and physical properties than the elements that it is made of. • Always a homogeneous sample • Can be broken down by a chemical change

Mixtures • Made up of 2 or more different substances physically combined • Can exist in variable ratio – There is no exact recipe to make salt water • The mixture will retain the physical and chemical properties of the substances that it is made of. • Can be homogeneous (consistent makeup) or heterogeneous (variable or inconsistent makeup) • Can be broken down by a physical change

Homogeneous Mixtures • A homogeneous mixture has the same uniform appearance and composition throughout. • Homogeneous mixtures are commonly referred to as solutions. • A solution is a homogeneous mixture of two or more substances in a single phase. • A mixture of gases is homogeneous • Alloy: a solid homogeneous solution of metals

Heterogeneous Mixture • A heterogeneous mixture consists of visibly different substances or phases. • Suspensions are heterogeneous mixture of larger particles. These particles are visible and will settle out on standing. Example: fine sand in water • colloid is a homogeneous solution with intermediate particle size between a solution and a suspension. Colloid particles may be seen in a beam of light such as dust in air in a "shaft" of sunlight. Milk, fog, and jello are examples of colloids.

Matter Substance • Homogeneous • Can write a • chemical formula Element • Only contains one type of atom Compound • Two or more different atoms chemically bonded Mixture • Variable ratio Homogeneous • Solutions • Gaseous mixtures • alloy Heterogeneous • Colloids • suspensions

What’s next…. • Particle diagrams…. how can we visually show elements, compounds, and mixtures (both heterogeneous and homogeneous) is a drawing. • In preparation for tomorrow please – be able to distinguish between a elements, compounds, molecules and mixtures (both heterogeneous and homogeneous) – Bring a few different color pencils or pens (at least 3)