What are some things that come to mind








- Slides: 16

What are some things that come to mind when you think about the word. . Chemistry

Chemistry � The study of the composition and structure of matter (anything that has mass) and the chemical reactions by which substances are changed into other substances. � Where did chemistry begin? ? ? • 100, 000 years ago (Earth Dwellers discovered fire) • Egyptian �used wine making (chemical fermentation process) �Metals �Dyes, glass, pottery, embalming fluids

Types of Observations � Visual Information • Colors, movement, light intensity, etc. . � Audible Information • Sounds, bubbling, fizzing, etc… � Olfactory Information • Smells, aromas, etc… � Tactile Information • Texture, hardness, temperature, etc…. Inferences � Inferences are hypotheses based on observations

Quantitative vs. Qualitative � Data that is based on quantities obtained using a quantifiable measurement process � This data is qua. Ntitative Descriptions or distinctions are based on some quality or Characteristic rather than on some quantity or measured value � This data is qualitative

Candle demo

Candle Question �How many of your observations were qualitative? �How many of your observations were quantitative? �Why do you think we performed this demo �What did you learn from this demo?

Practice Questions �Classify these statements qualitative or quantitative…. • The plant is short. • The candy was sour • The bus was 5 cm long

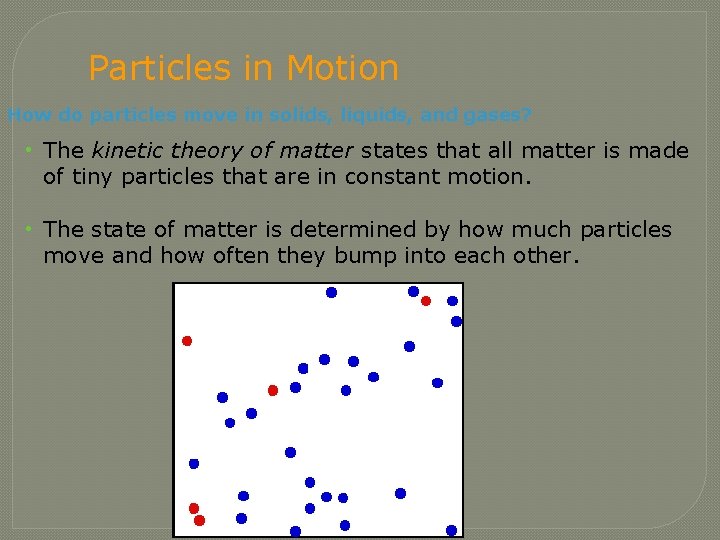
Particles in Motion How do particles move in solids, liquids, and gases? • The kinetic theory of matter states that all matter is made of tiny particles that are in constant motion. • The state of matter is determined by how much particles move and how often they bump into each other.

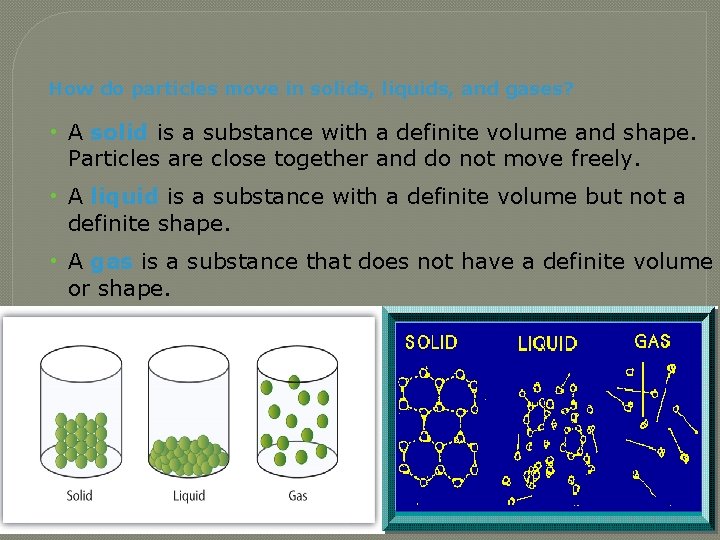
How do particles move in solids, liquids, and gases? • A solid is a substance with a definite volume and shape. Particles are close together and do not move freely. • A liquid is a substance with a definite volume but not a definite shape. • A gas is a substance that does not have a definite volume or shape.

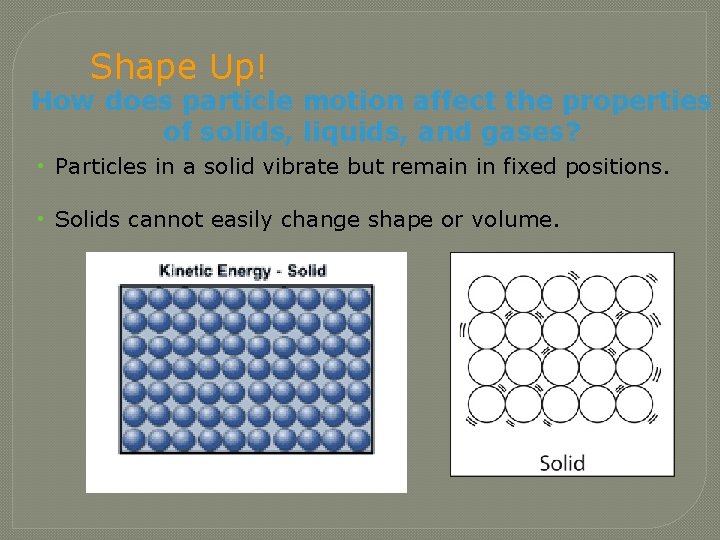
Shape Up! How does particle motion affect the properties of solids, liquids, and gases? • Particles in a solid vibrate but remain in fixed positions. • Solids cannot easily change shape or volume.

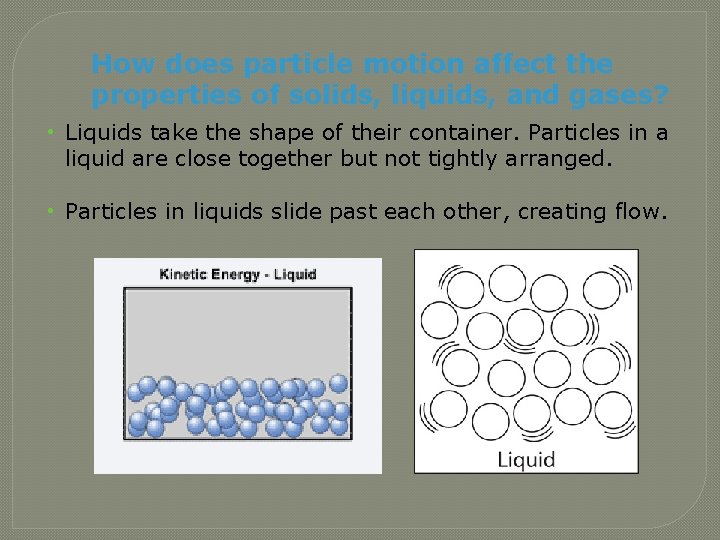
How does particle motion affect the properties of solids, liquids, and gases? • Liquids take the shape of their container. Particles in a liquid are close together but not tightly arranged. • Particles in liquids slide past each other, creating flow.

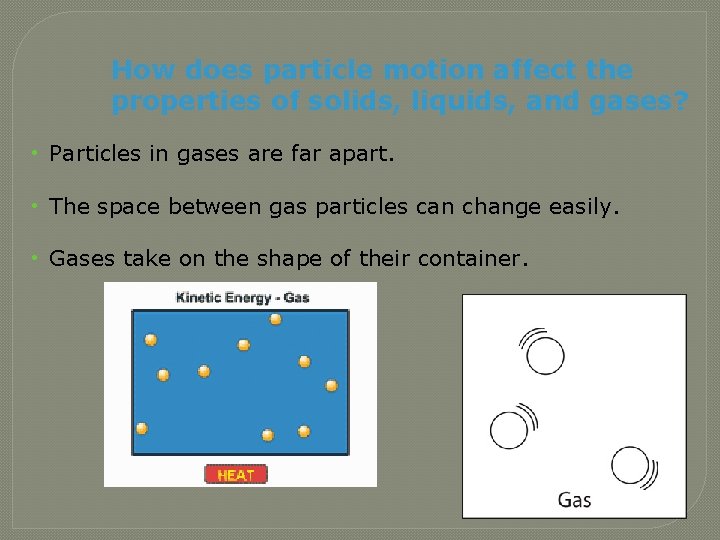
How does particle motion affect the properties of solids, liquids, and gases? • Particles in gases are far apart. • The space between gas particles can change easily. • Gases take on the shape of their container.

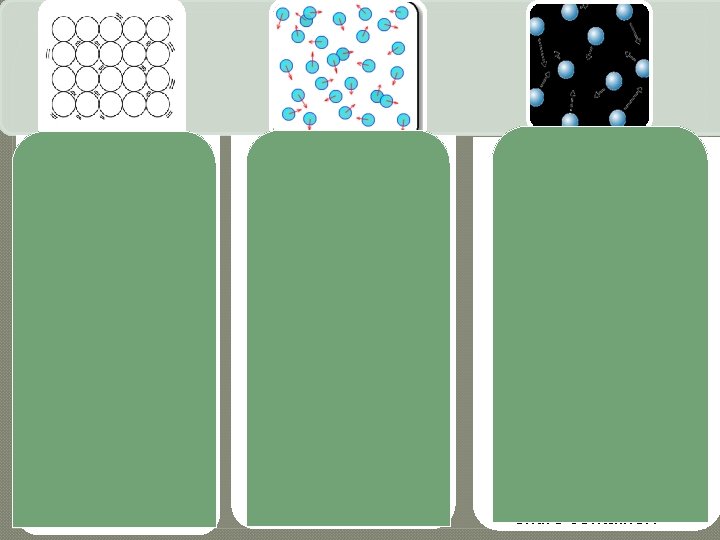
Solid • Definite volume and shape. • Particles vibrate. • Particles remain in fixed positions. • Particles are close together. • Cannot easily change shape or volume. Liquid • Definite volume but not a definite shape. • Liquids take the shape of their container. • Particles in a liquid are close together but not tightly arranged. • Particles in liquids slide past each other, creating flow. Gas • Do not have a definite volume or shape. • Particles in gases are far apart. • The space between gas particles can change easily. • Gases take on the shape of their container • Volume will not change; will always find a way to fill the entire container.

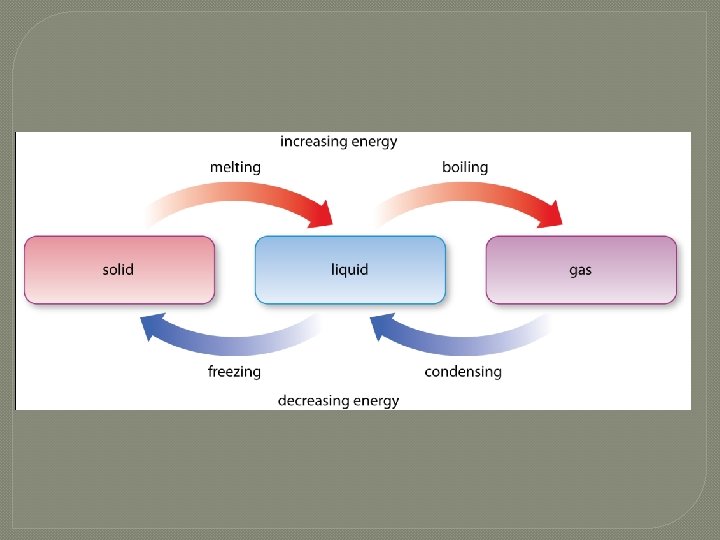
What happens when substances change state? • The process in which a solid becomes a liquid is called melting. • As a solid is heated, if the vibrations in the particles are fast enough, the particles break loose and slide past one another. • When temperatures of a liquid are lowered, causing a solid to form, it is called freezing. • Lower temperatures cause the particles to move slowly enough for the attractions between them to cause the liquid to become a solid. • Water freezes at 0°C, but other substances can freeze at room temperature. • When substances lose or gain energy, one of two things can happen to the substance: its temperature can change or its state can change.

What happens when substances change state? • When substances lose or gain energy, one of two things can happen to the substance: • its temperature can change • or its state can change.
