What are hydrocarbons Baseline Aiming for 4 Describe

What are hydrocarbons? Baseline (Aiming for 4): Describe the composition of crude oil. State a definition of a hydrocarbon. State a definition of an alkane. Further (Aiming for 6): Describe how to separate crude oil into fractions in a school laboratory. Classify a hydrocarbon as an alkane. State the names and describe the first four alkanes Challenge (Aiming for 8): Explain why fractional distillation is used to separate crude oil into fractions. Apply a general formula to generate a molecular formula and a displayed formula for a straight -chain alkane. Classify and justify the classification of a chemical as an alkane.

Homework teccscience. co. uk • Organic chemistry lesson 1 homework sheet • Either write the answers in your book, on paper, print the worksheet yourself or see me for a copy
Alkane Methane propane butane Molecular formula Displayed formula Molecular model
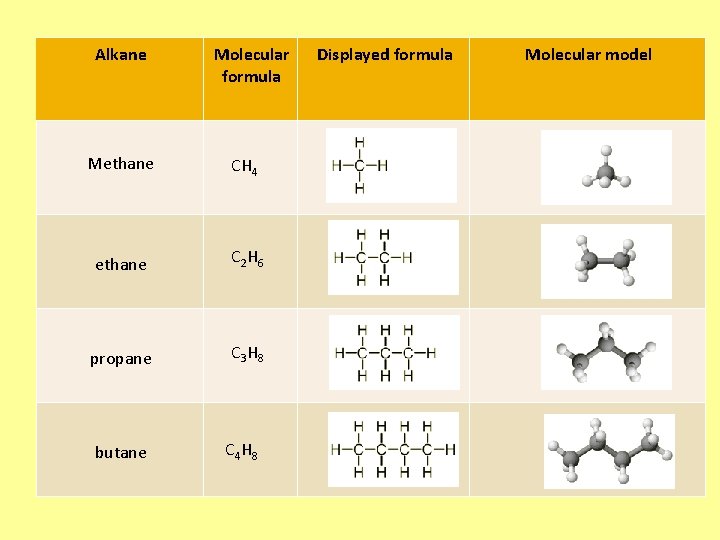
Alkane Molecular formula Methane CH 4 ethane C 2 H 6 propane C 3 H 8 butane C 4 H 8 Displayed formula Molecular model
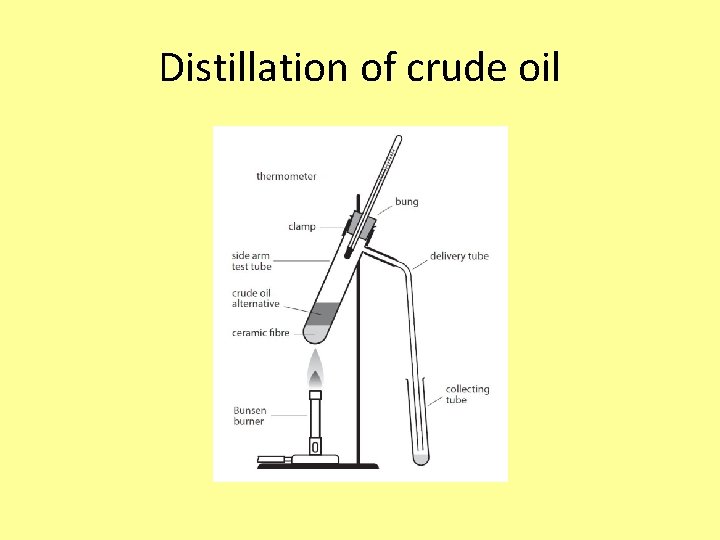
Distillation of crude oil

Exam question Crude oil is a mixture of many saturated hydrocarbons. They can be separated into fractions by the process of fractional distillation. State what is meant by: (i) hydrocarbon. . . . . . . . . . . . . . . (2) (ii) saturated. . . . . . . . . . . . . . . . (1) (iii) fraction. . . . . . . . . . . . . . . . (1) (Total 4 marks)

Mark scheme (i) carbon and hydrogen only or compound of for 1 mark each (ii) single bonds only or no double bonds etc or contains maximum number of hydrogen atoms for 1 mark (iii) molecules of similar chain length similar boiling points limited range of boiling points etc any 1 for 1 mark 2 1 1 [4]

• Diesel oil is obtained from crude oil. It can be used as a fuel for car engines. The diagram below represents a compound found in diesel oil. (a) What is the formula of this compound? . . . . . . . (1) (b) Each of the lines on the diagram above represents a covalent bond. What is a covalent bond? . . . . . . . . . . . . . . . . (2) (Total 3 marks)
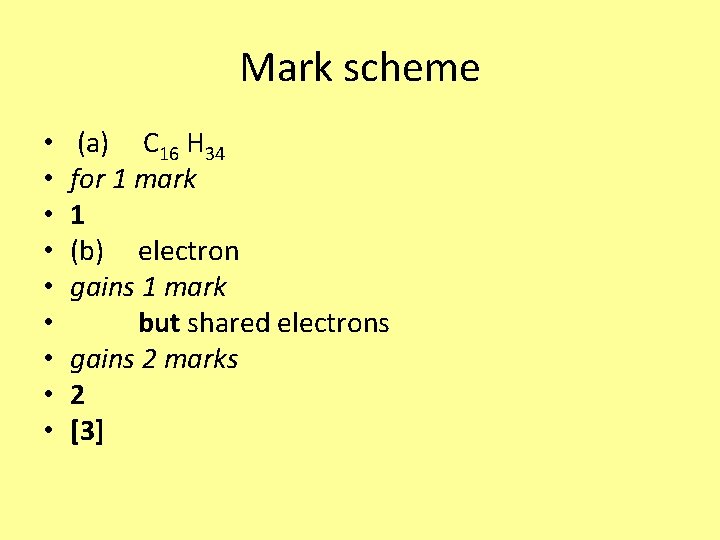
Mark scheme • • • (a) C 16 H 34 for 1 mark 1 (b) electron gains 1 mark but shared electrons gains 2 marks 2 [3]
- Slides: 9