What are Enzymes n n Most enzymes are






- Slides: 14

What are Enzymes? n n Most enzymes are proteins Enzymes help to speed up reactions

How do enzymes work? n n n Enzyme activity Each enzyme has a specific shape and function (lock and key theory) Enzymes help to make or break bonds They are re-usable. They are not destroyed in the reaction They decrease the energy needed in a reaction

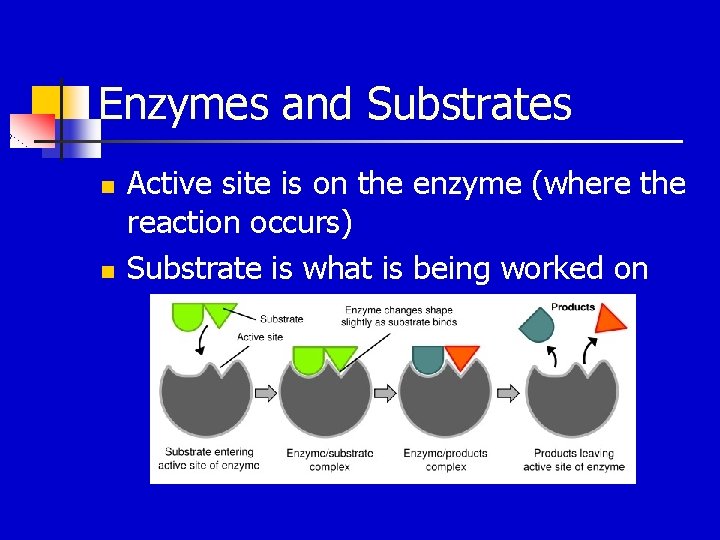
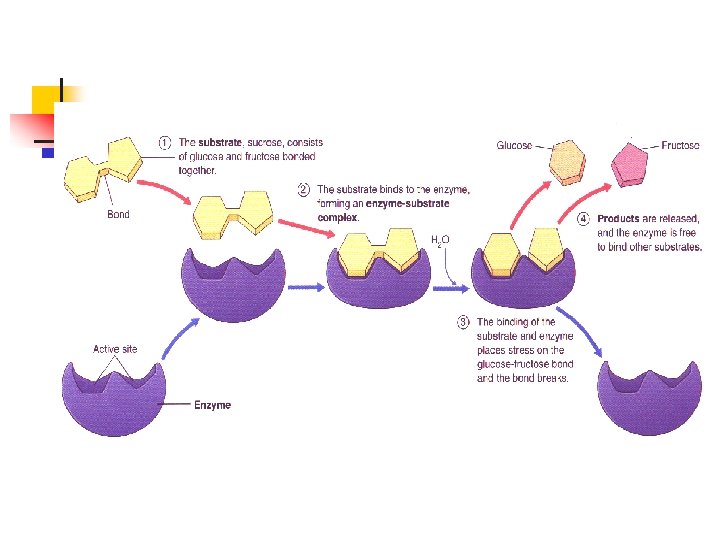
Enzymes and Substrates n n Active site is on the enzyme (where the reaction occurs) Substrate is what is being worked on

3 STEPS IN AN ENZYME REACTION n n n 1 - The enzyme and substrate(s) come together and join at the active site. 2 - The enzyme decreases the activation energy and bonds are either made or broken 3 - The product(s) are released and the enzyme is reused. It doesn’t get destroyed

LOCK AND KEY THEORY n n In order to open your door you must have the right shape key. Only substrates and enzymes that have the right shapes will fit together just like a lock and key or pieces of a puzzle.

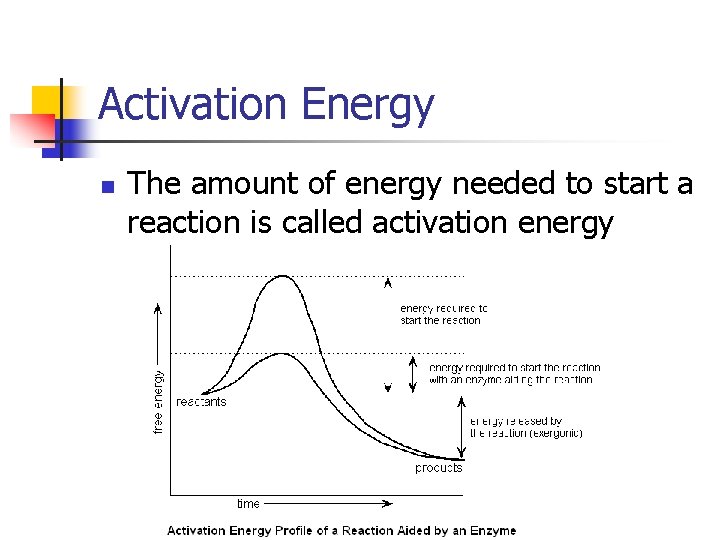
Activation Energy n The amount of energy needed to start a reaction is called activation energy

What can affect an enzyme’s function? n Too much heat n A change in the p. H n Both of these factors change the shape of the enzyme and make it nonfunctional. This is called Denatured

Factors that affect how fast the reactions occur n n The amount of the substrate The amount of the enzyme

What is a reaction? n You start with one molecule and you end up with something different Reactants – What you start with Products- What you end up with n Reactants Products n n

Examples n n CO 2 & H 2 O C 6 H 12 O 6 & O 2 Reactants energy Products The arrow indicates which direction the reaction is going. We are making glucose and oxygen All reactions require energy to get it started

The enzymes and the substrates move around until they collide

HOW DO ENZYMES WORK? n n n Enzymes have a space on them called an Active Site. This is where the reaction will take place. The substrate is what’s being changed fits into the active site It must be the right shape or it won’t work.

2 TYPES OF REACTIONS n n Therm = heat Exo = out Endo = in Exothermic n n n Heat comes out of the reaction You can feel heat being released Endothermic n n Heat is absorbed in the reaction You can feel it becoming colder
