What are enzymes Globular proteins made by living

What are enzymes? • Globular proteins • made by living cells for biochemical reactions – work inside and outside of cells • Living things produce THOUSANDS of enzymes – one enzyme catalyzes one biochemical reaction to start new ones Cascade effect
Analogy – SHOE HORN

The living cell is a miniature chemical factory where thousands of reactions occur

Metabolism = the chemical reactions within an organism • metabolic pathway - begins with a specific molecule and ends with a product • Each step is catalyzed by a specific enzyme Cascade effect
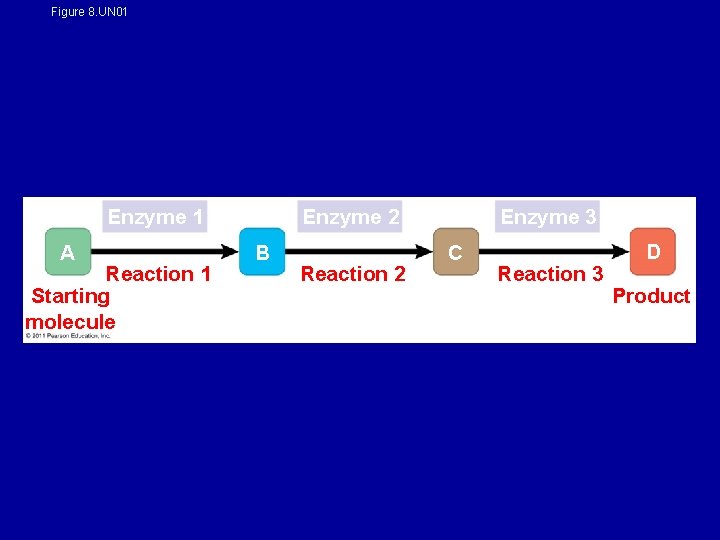
Figure 8. UN 01 Enzyme 2 Enzyme 1 A Reaction 1 Starting molecule B Reaction 2 Enzyme 3 C Reaction 3 D Product
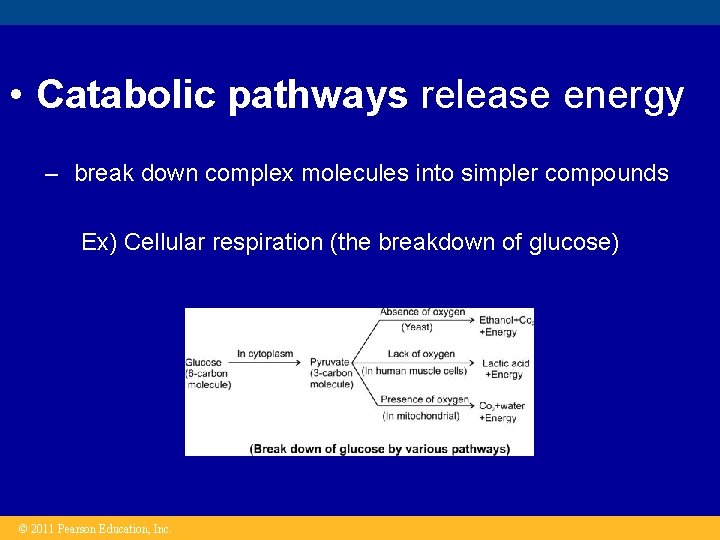
• Catabolic pathways release energy – break down complex molecules into simpler compounds Ex) Cellular respiration (the breakdown of glucose) © 2011 Pearson Education, Inc.
• Anabolic pathways consume energy – build complex molecules (from simpler ones) Ex) protein synthesis from amino acids • Bioenergetics - study of how organisms manage their energy resources © 2011 Pearson Education, Inc.

Let’s remember our Thermodynamics Laws! • 1 st law – – Energy cannot be created or destroyed it only be transferred or transformed • 2 nd law – – Every energy transfer increases the entropy of the universe – ENERGY FLOWS INTO AN ECOSYSTEM AS LIGHT AND EXISTS AS HEAT

Figure 8. 3 Heat Chemical energy (a) First law of thermodynamics (b) Second law of thermodynamics

The free-energy change of a reaction tells us whether or not the reaction occurs spontaneously • A living system’s free energy = energy that can do work
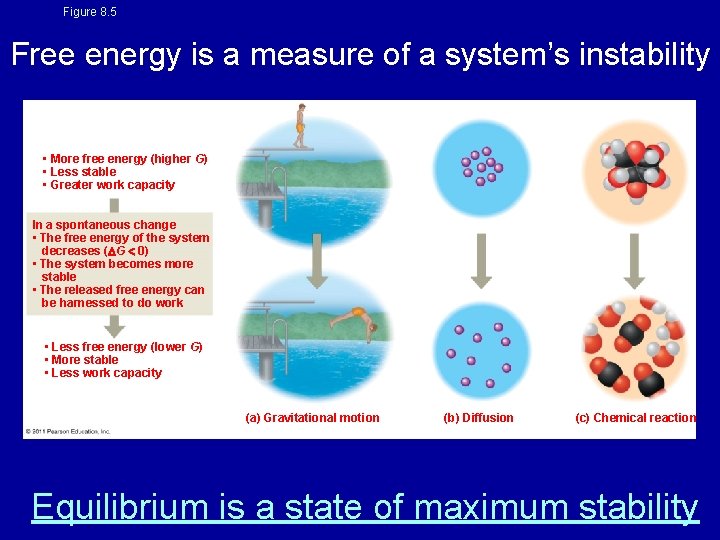
Figure 8. 5 Free energy is a measure of a system’s instability • More free energy (higher G) • Less stable • Greater work capacity In a spontaneous change • The free energy of the system decreases ( G 0) • The system becomes more stable • The released free energy can be harnessed to do work • Less free energy (lower G) • More stable • Less work capacity (a) Gravitational motion (b) Diffusion (c) Chemical reaction Equilibrium is a state of maximum stability

We can apply this concept of free energy to chemistry of life processes

Ender- and exergonic reaction – net release of free energy, spontaneous endergonic reaction – absorbs free energy from its surroundings, nonspontaneous

(a) Exergonic reaction: energy released, spontaneous Reactants Free energy Amount of energy released ( G 0) Energy Products Progress of the reaction (b) Endergonic reaction: energy required, nonspontaneous Products Free energy Figure 8. 6 Energy Reactants Progress of the reaction Amount of energy required ( G 0)

• cells manage energy resources by energy coupling (powered by ATP) – use of an exergonic process to drive an endergonic one
energy coupling in cells is mediated by ATP! • bonds between the P groups of ATP broken • hydrolysis • Energy released from ATP • release of energy comes from the chemical change to a state of lower free energy

Figure 8. 11 In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction ATP Energy from catabolism (exergonic, energy-releasing processes) ADP H 2 O Pi Driven by phosphorylation Energy for cellular work (endergonic, energy-consuming processes)
Why do we need enzymes? • Catalytic proteins – speeds up reactions • Necessary for metabolic processes – digestion, breathing, reproduction, coagulation of the blood, sensory perception all rely on enzymes • They are specific! • Lower the activation energy (Ea) of a reaction

Enzyme Role Pepsin Stomach enzyme used to break protein down into peptides. Lactase A digestive enzyme that breaks lactose into glucose and galactose. Topoisomerase A family of enzymes that act on the coiled structure of DNA. They cut the DNA to alter the coiled structure. Zymase A naturally occurring enzyme in yeasts, used in the baking industry to ferment sugar into ethanol and carbon dioxide.
The players in an enzymatic reaction… • Substrate – what the enzymes works on • Enzyme – the protein catalyst • Enzyme-substrate - complex of the 2 • Products • Active site – where substrate binds to enzyme

Life of a typical enzyme Enzyme Substrate Product
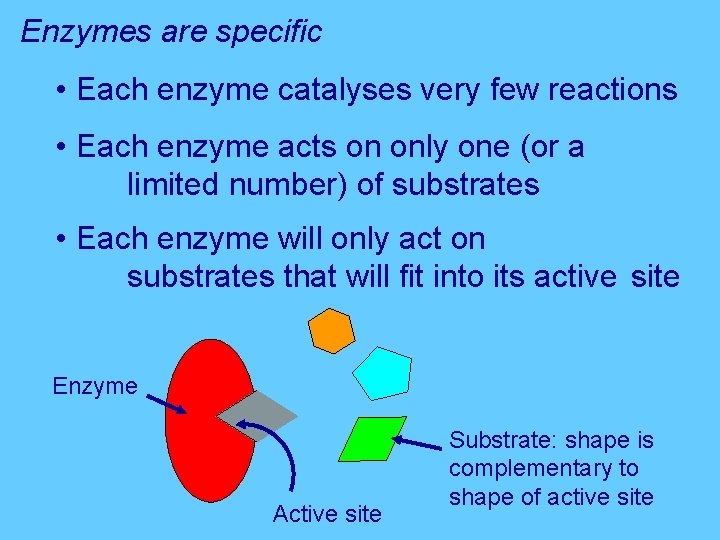
Enzymes are specific • Each enzyme catalyses very few reactions • Each enzyme acts on only one (or a limited number) of substrates • Each enzyme will only act on substrates that will fit into its active site Enzyme Active site Substrate: shape is complementary to shape of active site
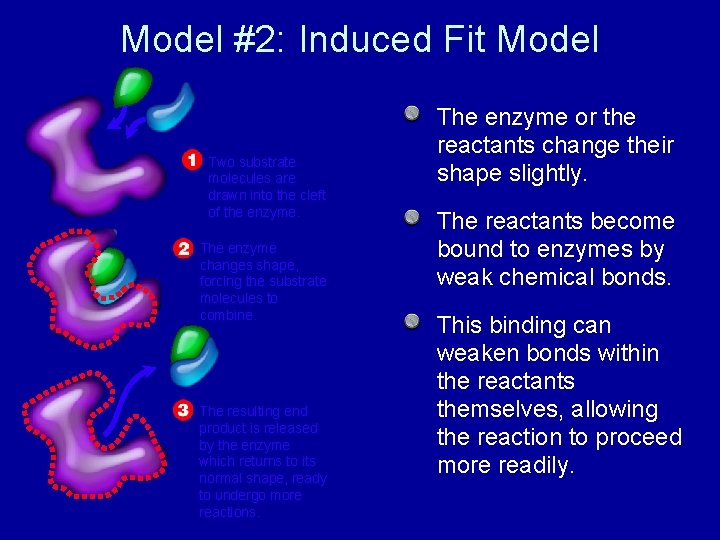
Model #2: Induced Fit Model Two substrate molecules are drawn into the cleft of the enzyme. The enzyme changes shape, forcing the substrate molecules to combine. The resulting end product is released by the enzyme which returns to its normal shape, ready to undergo more reactions. The enzyme or the reactants change their shape slightly. The reactants become bound to enzymes by weak chemical bonds. This binding can weaken bonds within the reactants themselves, allowing the reaction to proceed more readily.
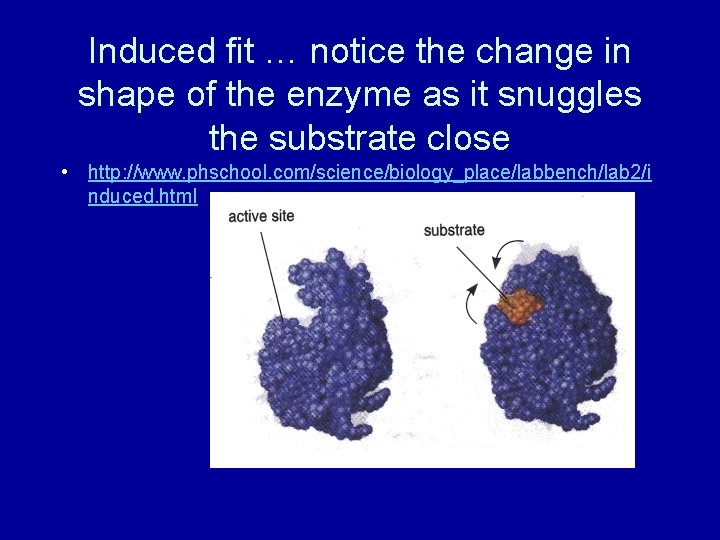
Induced fit … notice the change in shape of the enzyme as it snuggles the substrate close • http: //www. phschool. com/science/biology_place/labbench/lab 2/i nduced. html

Activation energy • Energy is needed to break chemical bonds Activation energy = energy which must be put into reactants to break some bonds - to get a reaction started

Activation energy – the energy needed to start a reaction/ energy needed to break bonds àWhat do you notice about the Ea? àWhere do you think the optimal temp is? àWhat about the energy?
Enzyme Cofactors • Nonprotein helpers that help catalyze reactions • Can either bind loosely or permanently on the protein • If the cofactor is organic, it is considered a coenzyme • Vitamins are cofactors
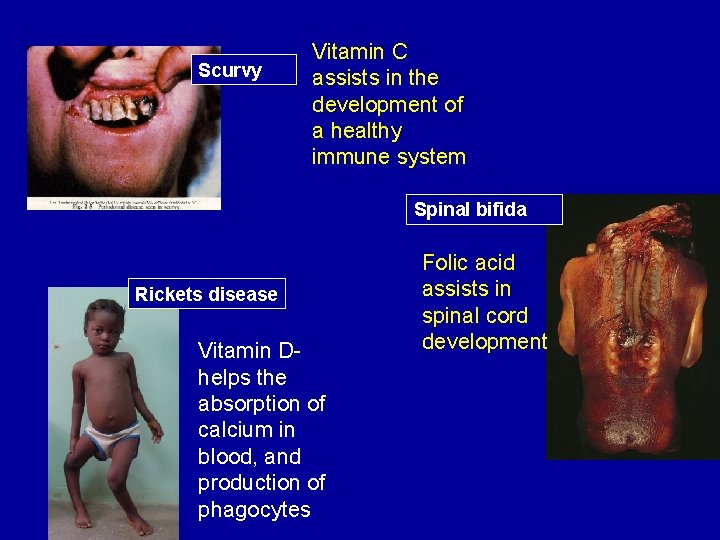
Scurvy Vitamin C assists in the development of a healthy immune system Spinal bifida Rickets disease Vitamin Dhelps the absorption of calcium in blood, and production of phagocytes Folic acid assists in spinal cord development

Enzyme Inhibitors Two types Competitive Non-competitive Many drug molecules are enzyme inhibitors.
Competitive Inhibitors • Resembles the substrates • Blocks substrates from binding to active sites of enzymes • If we increase the substrate concentration, will the rate of reaction increase? • YES! – the chance of an enzyme interacting with an enzyme goes up Example – penicillin – it blocks the enzyme of a bacteria involved in cell wall construction
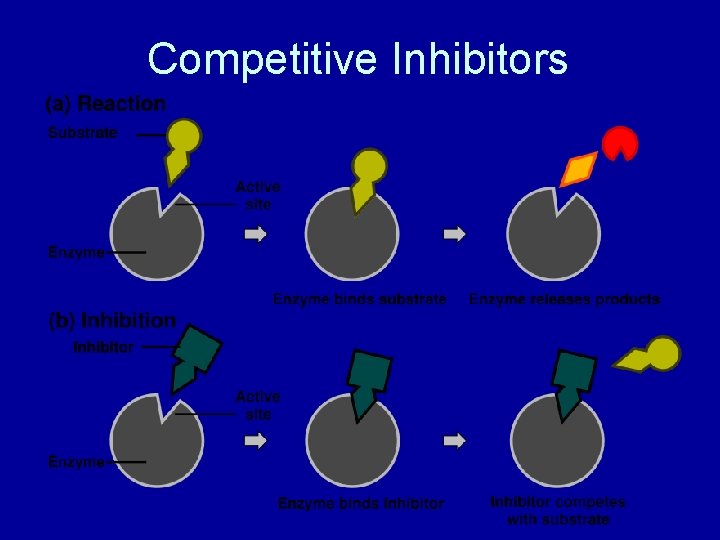
Competitive Inhibitors
Non-competitive inhibitors • Impede enzymatic reactions by binding on a part of the enzyme other than the active site • Causes enzyme to change shape. How does this affect catalytic reactions? • It can slow down, or won’t happen at all because the enzymes are basically denatured. • If you increase the concentration of substrate will the rate of reaction increase? • NO – the enzymes aren’t working to bind substrate!

Non-competitive inhibitors

Example of non-competitive inhibitor – Sarin nerve gas • Serine, found on the active site of acetylcholinesterase,
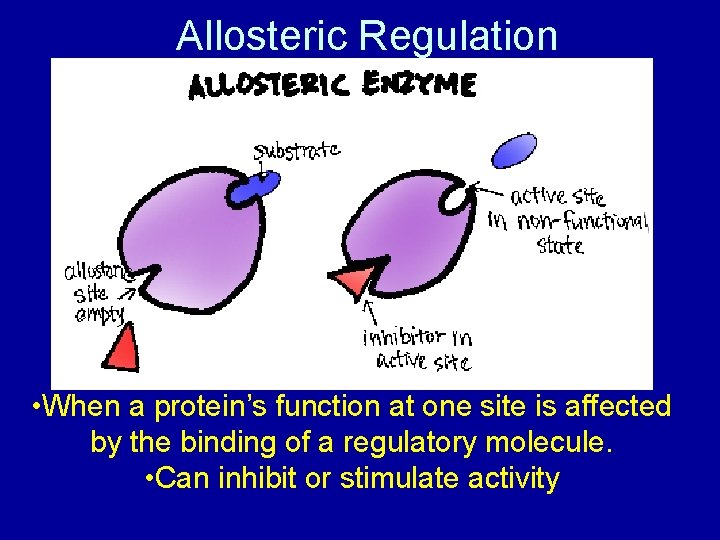
Allosteric Regulation • When a protein’s function at one site is affected by the binding of a regulatory molecule. • Can inhibit or stimulate activity

Allosteric Regulation: In Detail • An enzyme prone to allosteric regulation has 2 or more protein subunits – each subunit has an active site • If one unit changes, everything else does, too!
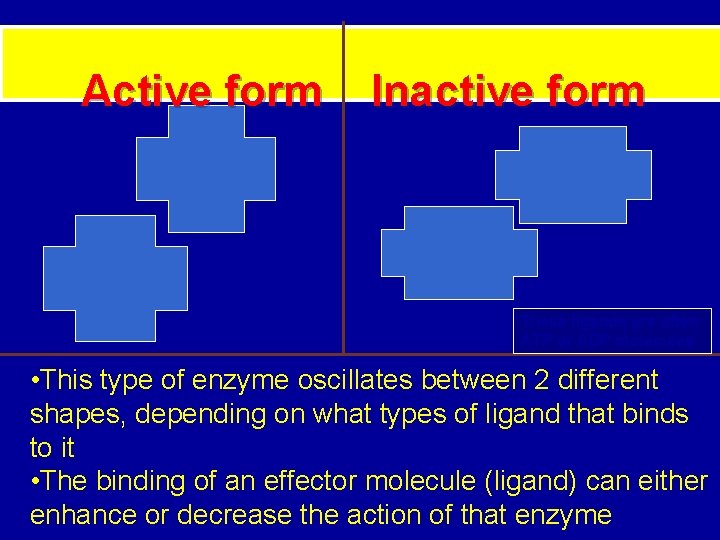
Active form Inactive form These ligands are often ATP or ADP molecules • This type of enzyme oscillates between 2 different shapes, depending on what types of ligand that binds to it • The binding of an effector molecule (ligand) can either enhance or decrease the action of that enzyme

Why do we need allosteric regulation? • Allosteric regulation makes sure that our body does not produce too much of one thing. • Feedback inhibition • The product itself attaches to the first enzyme in the chain and inhibits the chain of reactions until they need to produce more of that product. • http: //www. youtube. com/watch? v= M 5 bftq-W 2 a. Y
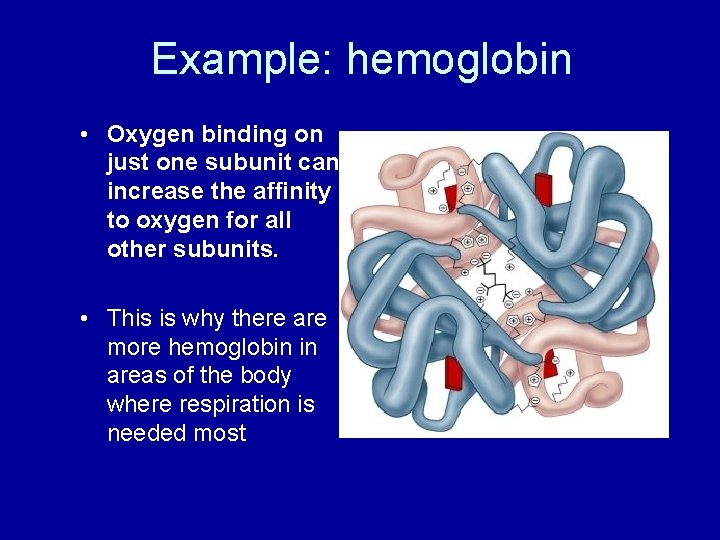
Example: hemoglobin • Oxygen binding on just one subunit can increase the affinity to oxygen for all other subunits. • This is why there are more hemoglobin in areas of the body where respiration is needed most

Enzyme of focus: Lactase

How does lactase work? • Pei, a twenty-six year old Asian graduate student, reached a new peak level of discomfort last Thursday evening about an hour after eating a cheeseburger and a large chocolate milk shake. • So, she went to the doctor. • Pei was given a lactose rich fluid to drink and had her blood glucose level measured several times over the course of two hours. Later, her doctor informed Pei that her blood glucose level had not risen after drinking the lactose rich fluid. • What do you think is going on here? What are they testing?

How does lactase work? • Lactase breaks down lactose sugar so that the simple sugar can be digested • Lactose is a large dissacharide, Lactase is an enzyme that specifically breaks down lactose sugar.

Case Study: Lactose Intolerance TOK: How has lactose intolerance affect the human population? How have scientists exploit what we know about enzyme substrate specificity to address lactose intolerance medical issues? • Hypothesis: original human species started out as lactose intolerant. The gene mutated for easier digestion - So…. Human’s diet might have been originally herbivoric, must gradually became omnivores. Therefore, we no longer had to rely on plants in the winter - our diets expanded. • 60% of most people of Asians and African decent stopped production of lactase. (maybe higher) • A study found that of 61% patients were LI, 2% Denmark, rest in Zambia - LI increases with latitude and with rising temperatures • Adults don’t produce enough lactase with time. • Although 1 st dairy farmers were from Europe, about 80% are LI. • LI is genetically based, oral supply of LT gene may be available. • Alternative method: consumption of Kefir, a set of bacteria that can help breakdown lactose.

Exit Tasks (1) On Index card: – Define Enzyme – Define Active Site – Define Enzyme Specificity and give an example. – State one question you have about what we learned today

Videos • https: //www. youtube. com/watch? v=c 5 j 6 Ex. HLFD 8 (overview of enzymes) • https: //www. youtube. com/watch? v=yk 14 d. OOvw. Mk (how enzymes work)
- Slides: 46