What Are Carbohydrates Produced by plants during photosynthesis

What Are Carbohydrates? • Produced by plants during photosynthesis • After eating plant foods, humans convert the carbohydrates into glucose • Glucose – Most abundant carbohydrate – Preferred source of energy for the blood, brain, and nervous system • Carbohydrate-rich plant foods make up the foundation of diets all over the world

• Made of carbon, hydrogen, and oxygen in a 1: 2: 1 ratio • Primary fuel source for body cells • Divided into two main classes: • Simple sugars • Complex sugars
Classification of Carbohydrates • Simple carbohydrates – Monosaccharide – Disaccharide – Perceived as sweeter than complex carbohydrates • Mixes with saliva and reacts with taste buds • Oligosaccharides • Complex carbohydrates – Polysaccharides
Monosaccharides • Glucose – Blood glucose and blood sugar in the body – Most abundant monosaccharide in the body • Is the preferred and main source of energy for the brain and red blood cells – Part of every disaccharide – Only monosaccharide in starches
Monosaccharides • Three nutritionally important monosaccharides – Glucose – Fructose – Galactose
Monosaccharides: Single Sugars Glucose – carbohydrate form used by the body, referred to as “blood sugar” – basic sub-unit of other larger carbohydrate molecules – found in fruits, vegetables, honey 6
• Fructose – Sweetest of natural sugars – Found abundantly in fruits – Part of high-fructose corn syrup • Galactose – Commonly occurs as part of dissaccharide lactose
Fructose – sweetest of the sugars – occurs naturally in fruits & honey, “fruit sugar” – combines with glucose to form sucrose Galactose – combines with glucose to form lactose, “milk sugar” 8
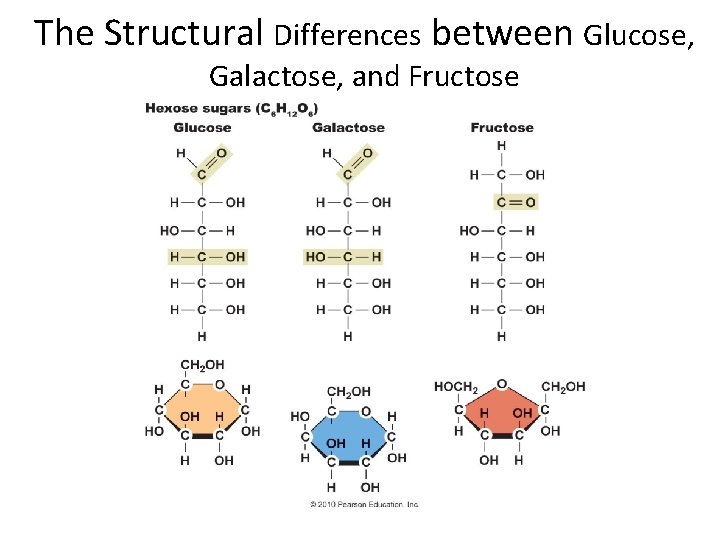
The Structural Differences between Glucose, Galactose, and Fructose
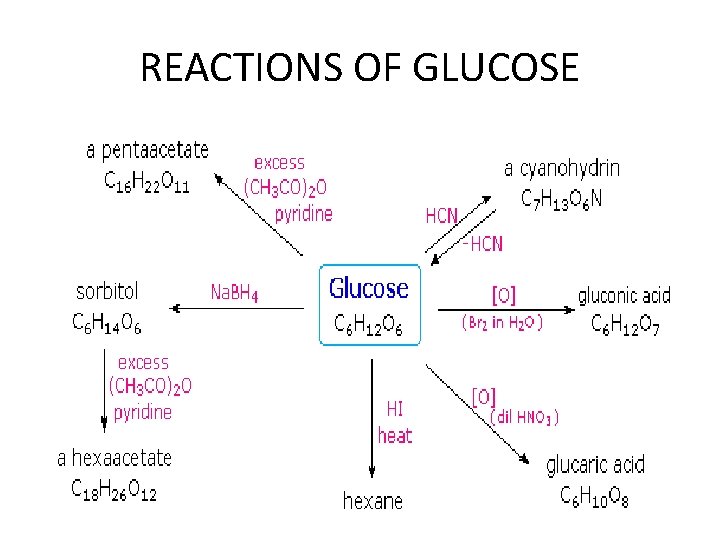
REACTIONS OF GLUCOSE
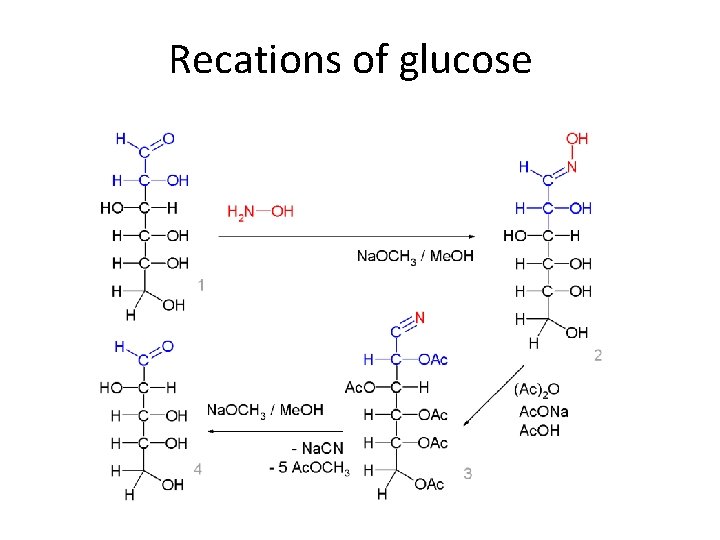
Recations of glucose
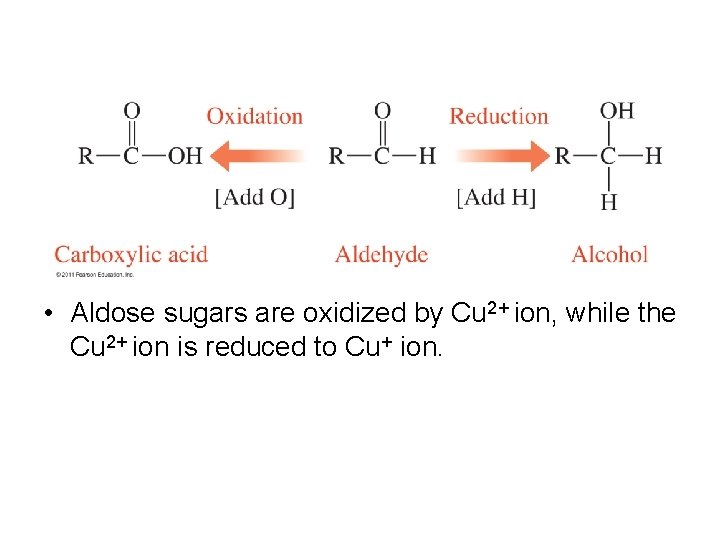
• Aldose sugars are oxidized by Cu 2+ ion, while the Cu 2+ ion is reduced to Cu+ ion.
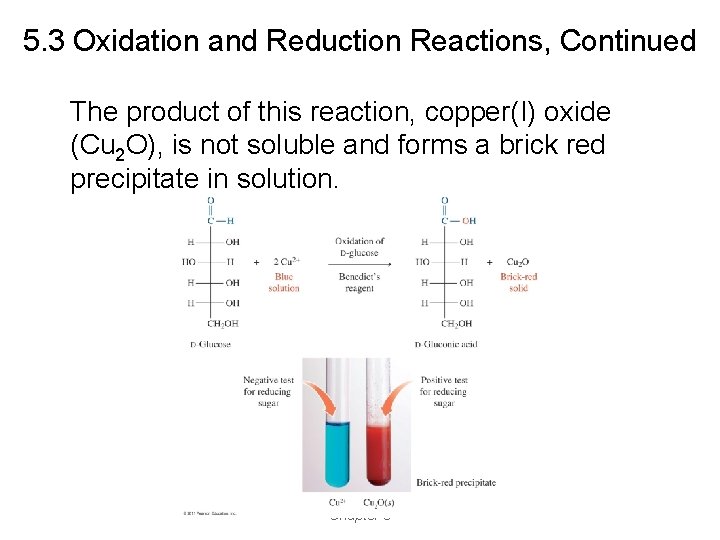
5. 3 Oxidation and Reduction Reactions, Continued The product of this reaction, copper(I) oxide (Cu 2 O), is not soluble and forms a brick red precipitate in solution. Chapter 5
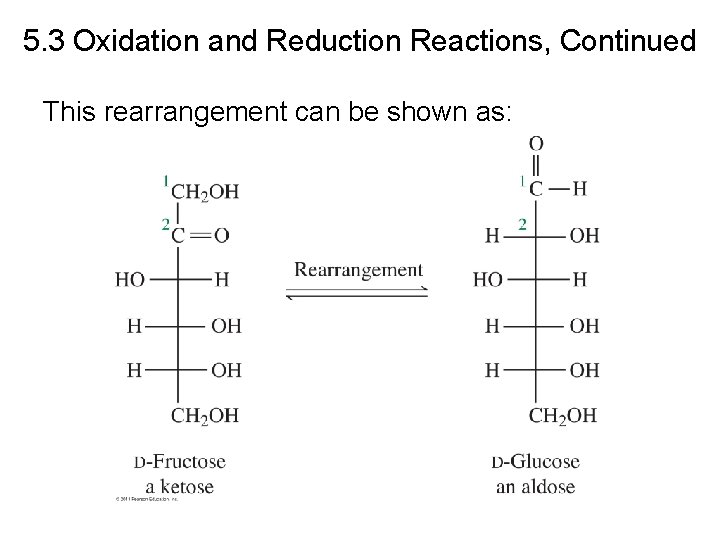
5. 3 Oxidation and Reduction Reactions, Continued This rearrangement can be shown as:
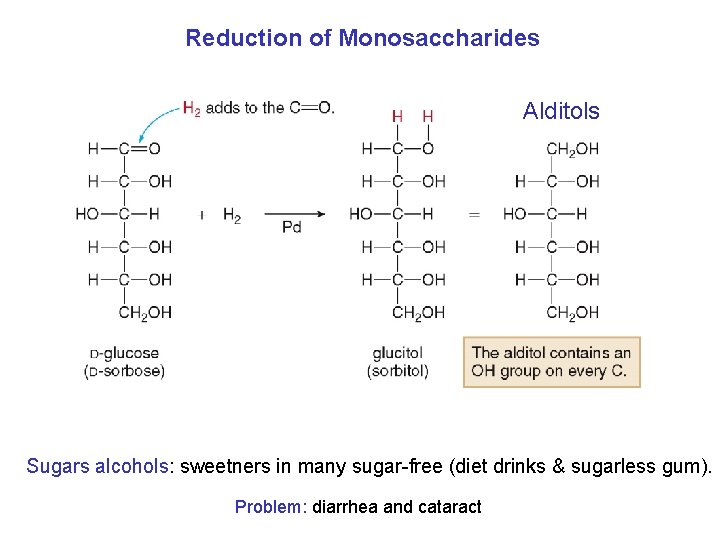
Reduction of Monosaccharides Alditols Sugars alcohols: sweetners in many sugar-free (diet drinks & sugarless gum). Problem: diarrhea and cataract
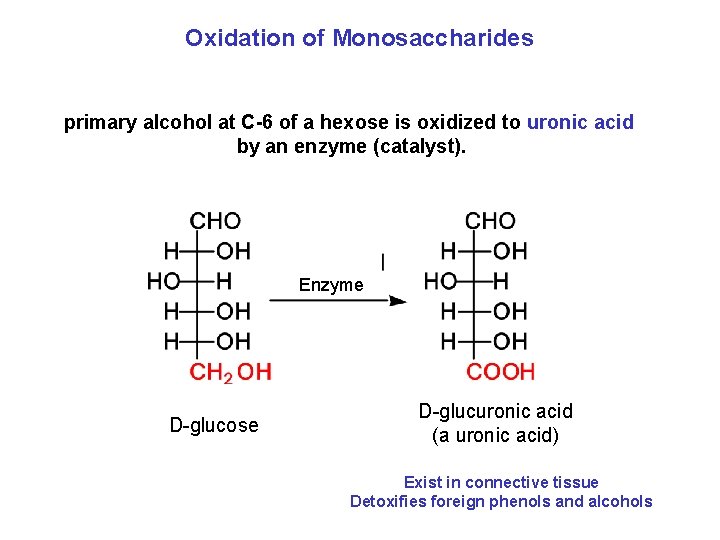
Oxidation of Monosaccharides primary alcohol at C-6 of a hexose is oxidized to uronic acid by an enzyme (catalyst). Enzyme D-glucose D-glucuronic acid (a uronic acid) Exist in connective tissue Detoxifies foreign phenols and alcohols
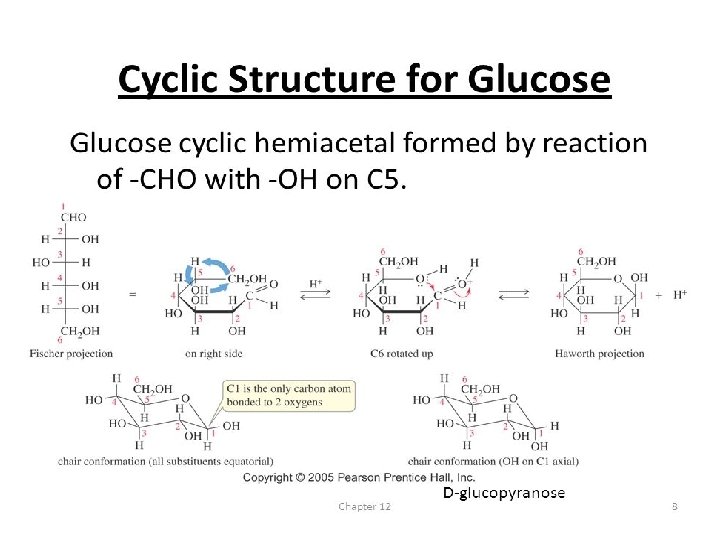
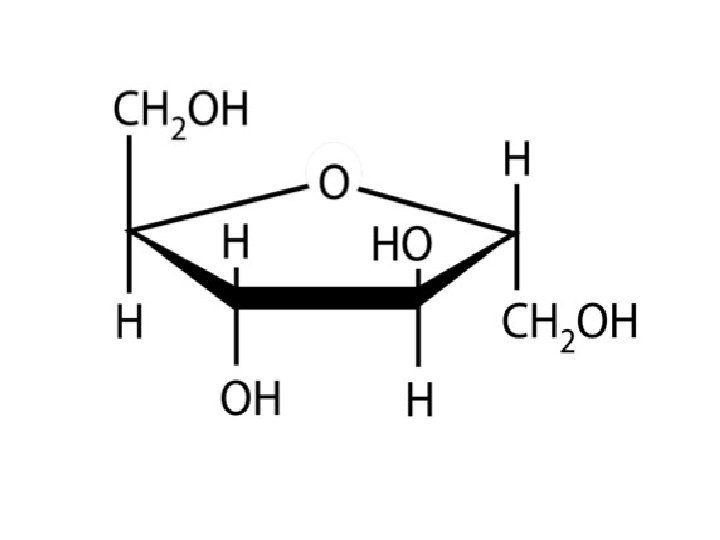

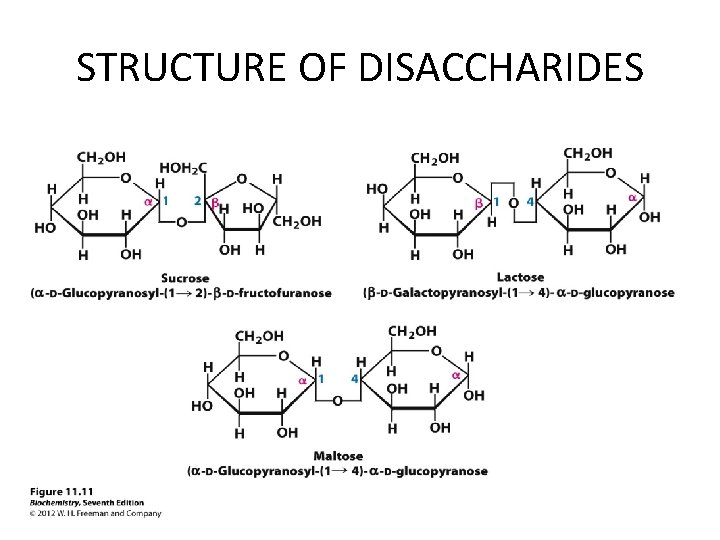
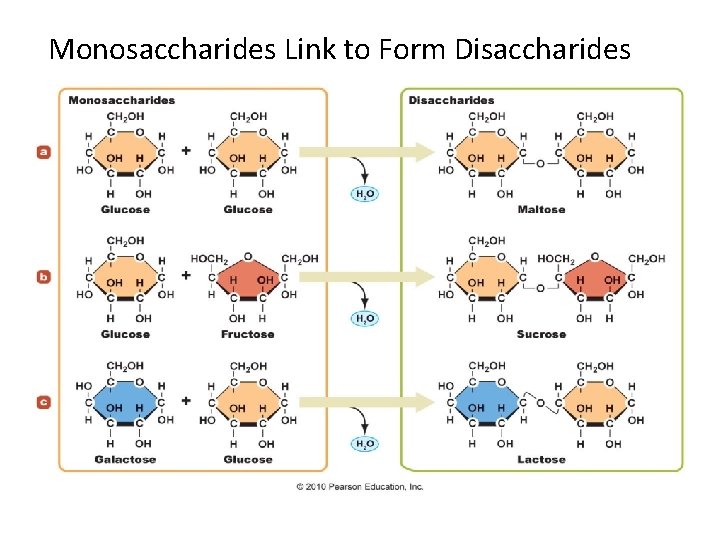
Disaccharides • Three Disaccharides – Sucrose • Most common – Lactose – Maltose • Least common • Formed from digestion of starches
STRUCTURE OF DISACCHARIDES
Monosaccharides Link to Form Disaccharides
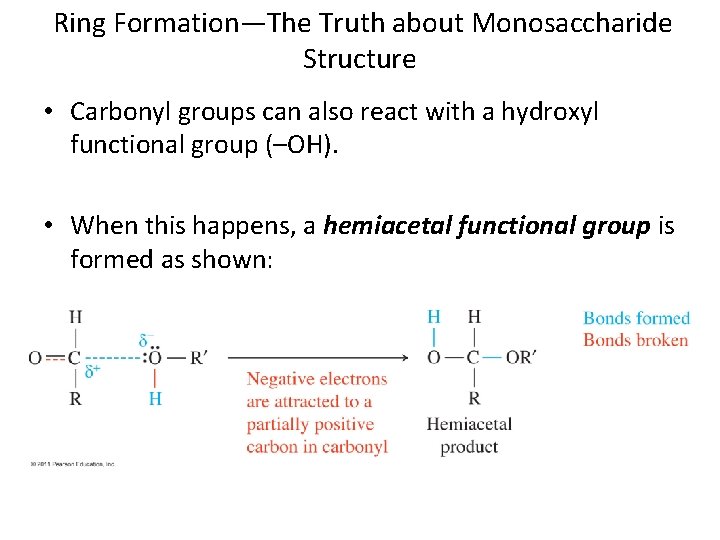
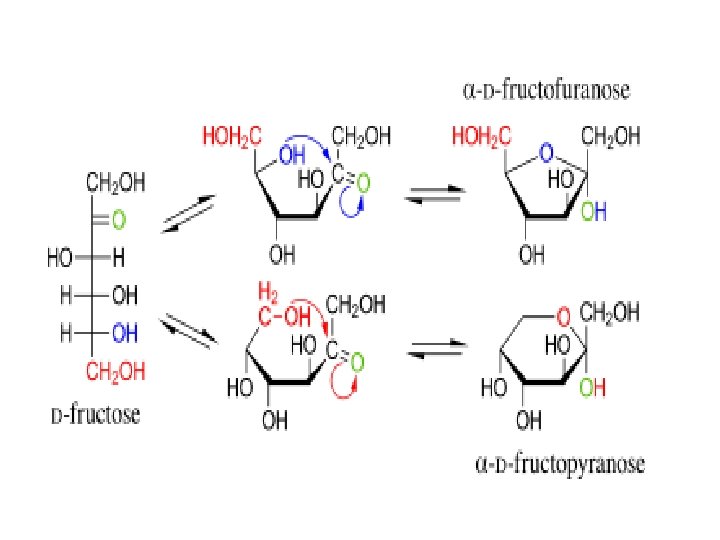
Ring Formation—The Truth about Monosaccharide Structure • Carbonyl groups can also react with a hydroxyl functional group (–OH). • When this happens, a hemiacetal functional group is formed as shown:
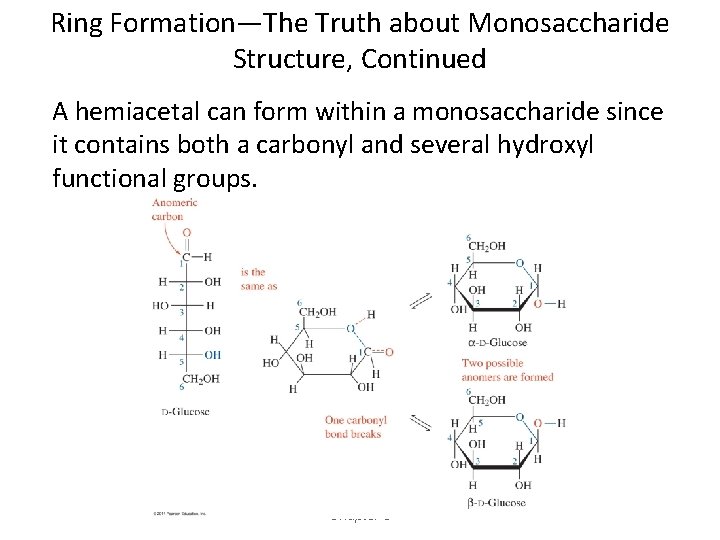
Ring Formation—The Truth about Monosaccharide Structure, Continued A hemiacetal can form within a monosaccharide since it contains both a carbonyl and several hydroxyl functional groups. Chapter 5

Ring Formation—The Truth about Monosaccharide Structure, Continued • The carbonyl carbon that reacts to form the hemiacetal is referred to as the anomeric carbon. • Two ring arrangements can be produced. These are termed anomers, and are referred to as the alpha ( ) and beta (β) anomer. • The position of the –OH group on the anomeric carbon relative to the position of the carbon outside the ring determines the type of anomer present.
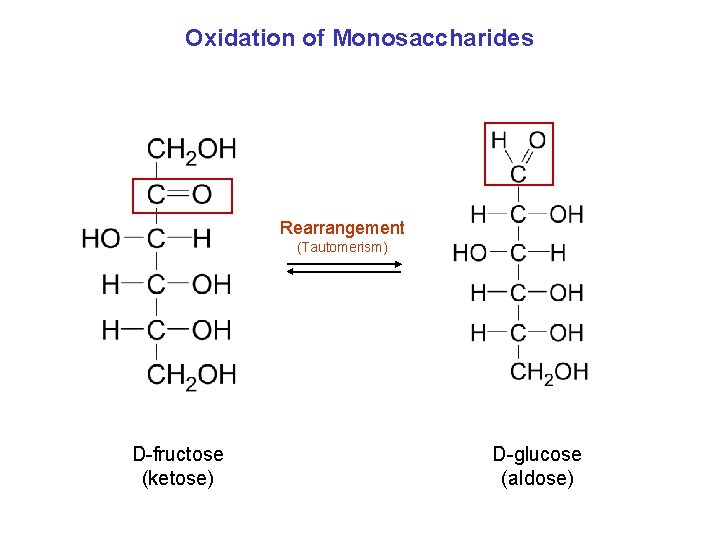
Oxidation of Monosaccharides Rearrangement (Tautomerism) D-fructose (ketose) D-glucose (aldose)

Oligosaccharides • Similar in length to simple carbohydrates • Similar in makeup to polysaccharides • Humans lack the enzymes necessary to digest them • Intestinal microflora digest and ferment them – Cause bloating, discomfort, and flatulence • Food sources – Legumes, beans, cabbage, brussels sprouts, broccoli
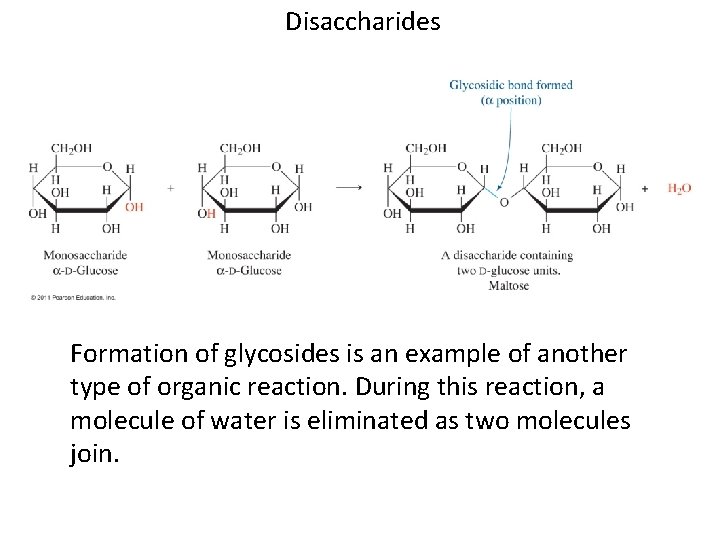
Disaccharides Formation of glycosides is an example of another type of organic reaction. During this reaction, a molecule of water is eliminated as two molecules join.
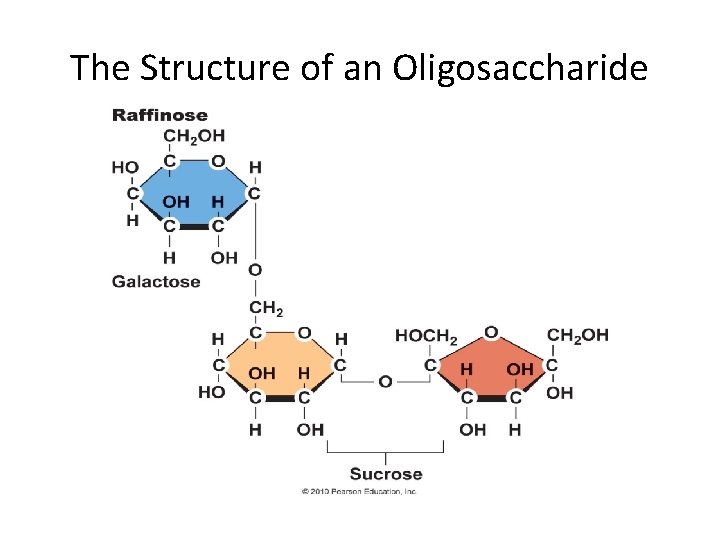
The Structure of an Oligosaccharide

Lactose • Lactose is known as milk sugar. • It is found in milk and milk products. • An intolerance to lactose can occur in people who inherit or lose the ability to produce the enzyme lactase that hydrolyzes lactose into its monosaccharide units. • The glycosidic bond is (1→ 4). • One of the anomeric carbons is free, so lactose is a reducing sugar.
POLYSACCHARIDES • Digestible polysaccharides: – Starch • Amylose • Amylopectin – Glycogen • Non-digestible polysaccharides: fibers – Soluble fiber – Insoluble fiber
Storage Polysaccharides Amylose and amylopectin—starch • Starch is a mixture of amylose and amylopectin and is found in plant foods. • Amylose makes up 20% of plant starch and is made up of 250– 4000 D-glucose units bonded α(1→ 4) in a continuous chain. • Long chains of amylose tend to coil. • Amylopectin makes up 80% of plant starch and is made up of D-glucose units connected by α(1→ 4) glycosidic bonds.

• Starch – Plants store glucose in chains of starch • Amylose – Straight chain – More resistant to digestion – Resistant starch ü May improve health of digestive tract ü May improve glucose tolerance ü May stimulate growth of beneficial intestinal bacteria • Amylopectin – Branched chains – Easier to digest
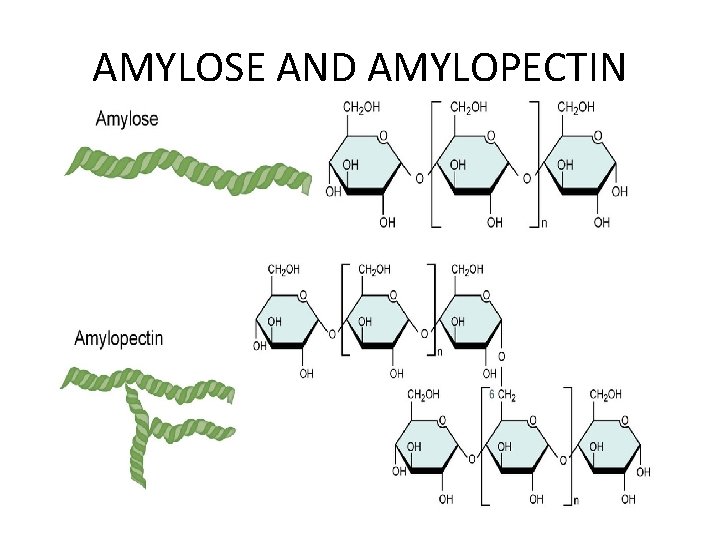
AMYLOSE AND AMYLOPECTIN

CELLULOSE Cellulose • Cellulose is an insoluble fiber in our diet because we lack the enzyme cellulase to hydrolyze the (1→ 4) glycosidic bond. • Whole grains are a good source of cellulose. • Cellulose is important in our diet because it assists with digestive movement in the small and large intestine. • Some animals and insects can digest cellulose because they contain bacteria that produce cellulase.
• Fiber – Nondigestible polysaccharides – Provides no energy – Classification • Soluble – Pectins, beta-glucan, some gums, mucilage – Easily fermented by intestinal bacteria • Carbon dioxide, methane, some fatty acids • Insoluble – Cellulose, lignin, some hemicelluloses – Not easily fermented

• Glycogen – Storage form of glucose in animals – Long, branched chains of glucose – Stored in liver and muscle – Liver glycogen response to blood glucose (BG) levels BG glycogen breakdown BG – Muscle glycogen can be broken down for energy for the muscle

Functions of Carbohydrates 1) Energy • glucose fuels the work of most of the body’s cells – preferred fuel of NERVOUS TISSUE (the brain, nerves) and RED BLOOD CELLS (RBC) • excess glucose is stored as GLYCOGEN in liver and muscle tissue 38

2) Sparing Body Protein • if diet does not provide enough glucose, then other sources of glucose must be found • if carbohydrate intake < 50 - 100 g, body protein will be used to make glucose • an adequate supply of carbohydrate spares body proteins from being broken down to synthesize glucose 39

3) Preventing Ketosis (Anti-ketogenic) • carbohydrates required for the complete metabolism of fat • incomplete fat metabolism produces KETONES • an adequate supply of carbohydrate (> 50 – 100 g per day) prevents KETOSIS 40

Thank You MR R. P. JHARIA K. V. V. F. JABALPUR
- Slides: 41