What are atoms elements molecules and compounds Atoms
- Slides: 12

What are atoms, elements, molecules, and compounds?

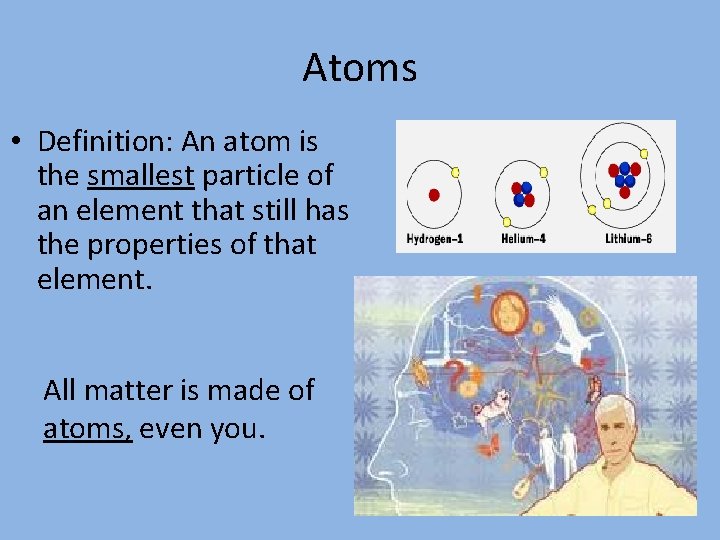
Atoms • Definition: An atom is the smallest particle of an element that still has the properties of that element. All matter is made of atoms, even you.

Atoms • Atoms are structures too small for us to see, even with aid of a microscope. • Because we can’t see atoms, we use models to help us study and understand them

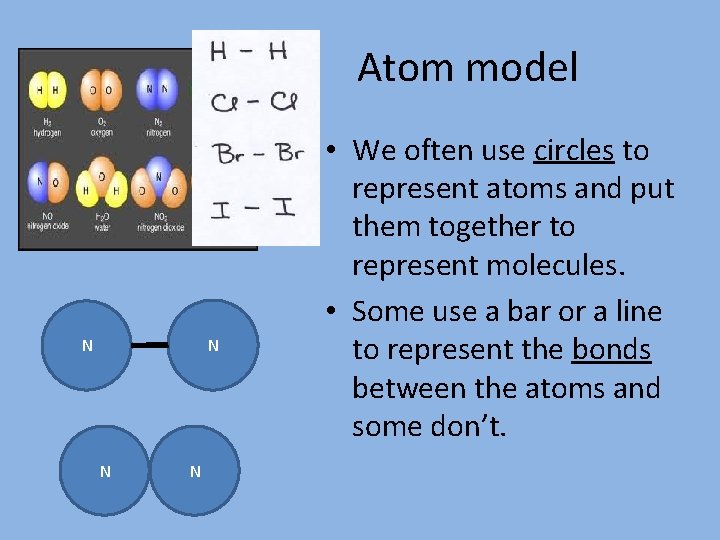
Atom model N N • We often use circles to represent atoms and put them together to represent molecules. • Some use a bar or a line to represent the bonds between the atoms and some don’t.

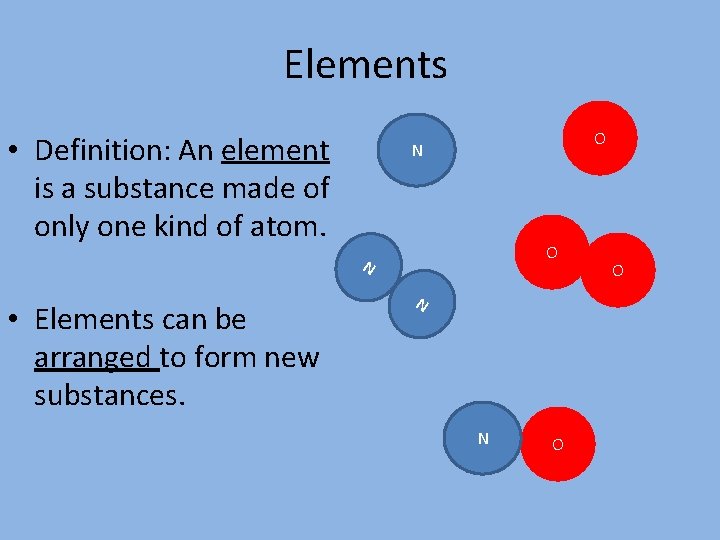
Elements • Definition: An element is a substance made of only one kind of atom. O N • Elements can be arranged to form new substances. N N O O

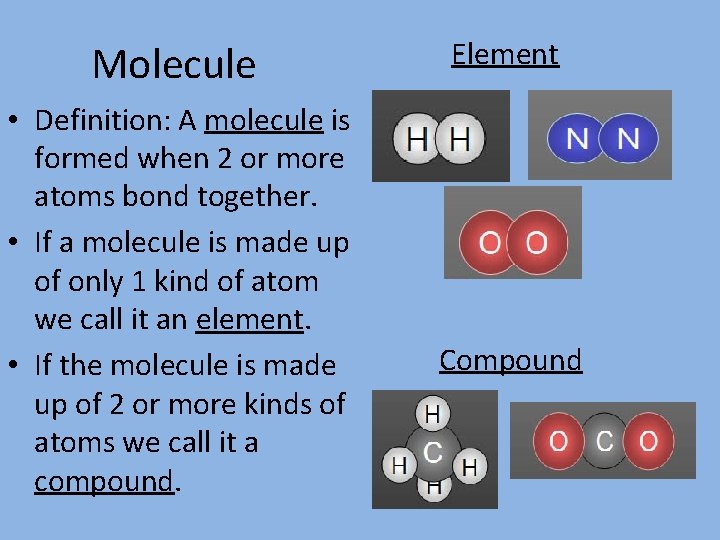
Molecule • Definition: A molecule is formed when 2 or more atoms bond together. • If a molecule is made up of only 1 kind of atom we call it an element. • If the molecule is made up of 2 or more kinds of atoms we call it a compound. Element Compound

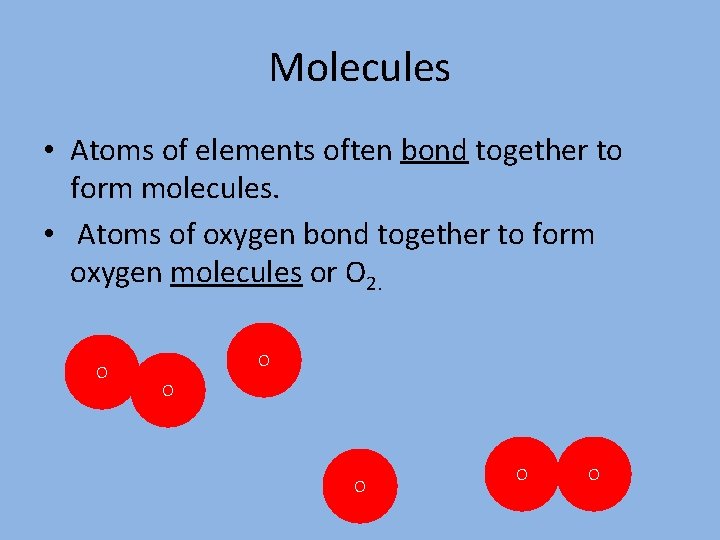
Molecules • Atoms of elements often bond together to form molecules. • Atoms of oxygen bond together to form oxygen molecules or O 2. O O O

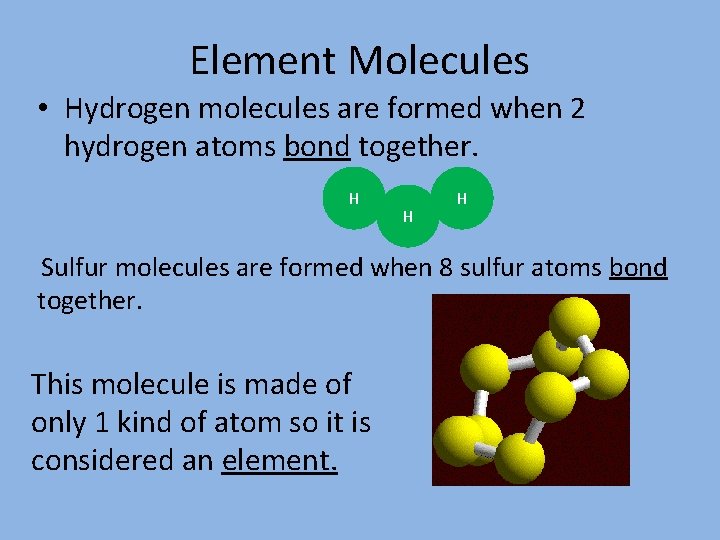
Element Molecules • Hydrogen molecules are formed when 2 hydrogen atoms bond together. H H H Sulfur molecules are formed when 8 sulfur atoms bond together. This molecule is made of only 1 kind of atom so it is considered an element.

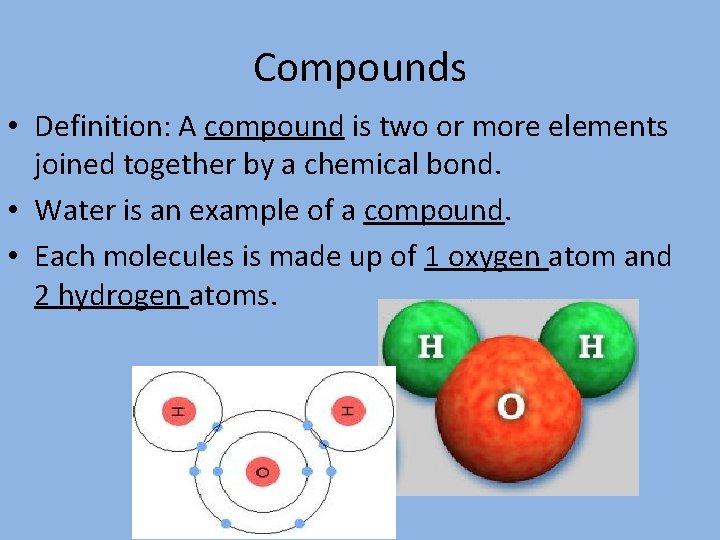
Compounds • Definition: A compound is two or more elements joined together by a chemical bond. • Water is an example of a compound. • Each molecules is made up of 1 oxygen atom and 2 hydrogen atoms.

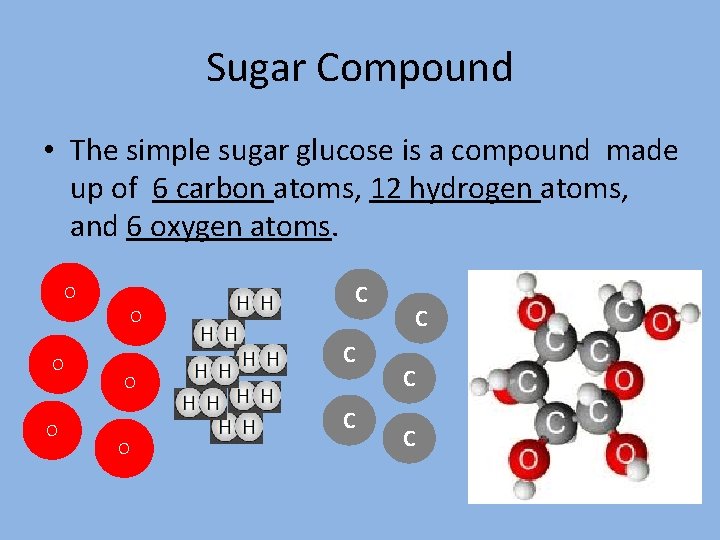
Sugar Compound • The simple sugar glucose is a compound made up of 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. O O C C C

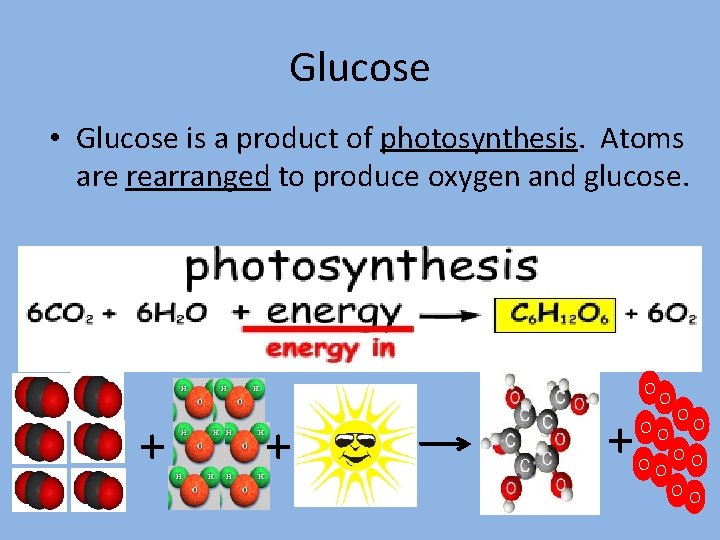
Glucose • Glucose is a product of photosynthesis. Atoms are rearranged to produce oxygen and glucose. O + + + O OO OO OO

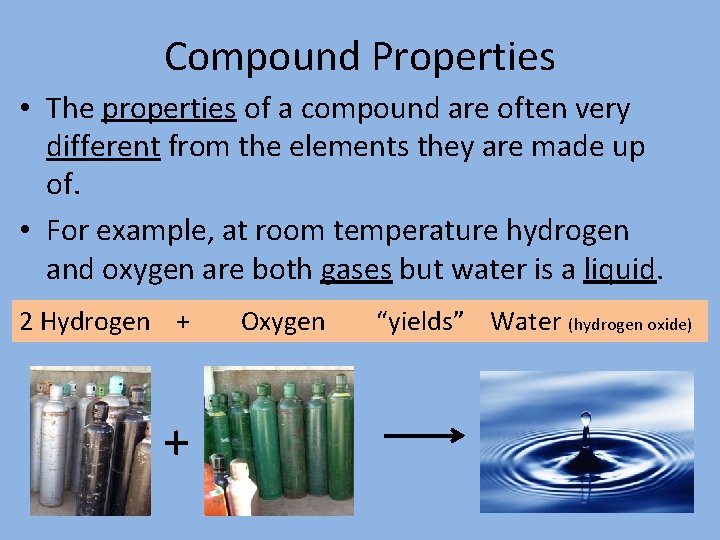
Compound Properties • The properties of a compound are often very different from the elements they are made up of. • For example, at room temperature hydrogen and oxygen are both gases but water is a liquid. 2 Hydrogen + + Oxygen “yields” Water (hydrogen oxide)