What are ATMPs From basic to regulatory definitions

What are ATMPs? From basic to regulatory definitions Heather Main - 4 th May 2021
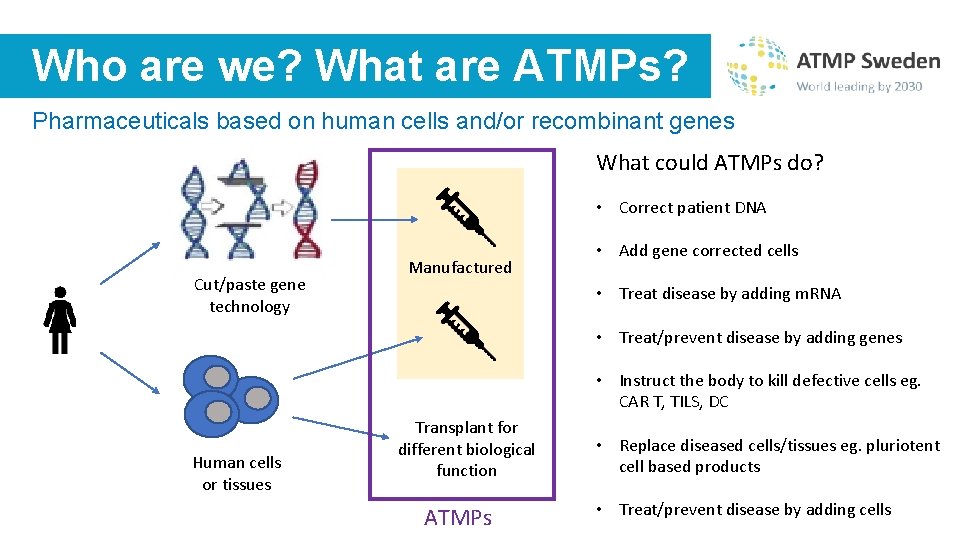
Who are we? What are ATMPs? Pharmaceuticals based on human cells and/or recombinant genes What could ATMPs do? • Correct patient DNA Cut/paste gene technology Manufactured • Add gene corrected cells • Treat disease by adding m. RNA • Treat/prevent disease by adding genes • Instruct the body to kill defective cells eg. CAR T, TILS, DC Human cells or tissues Transplant for different biological function ATMPs • Replace diseased cells/tissues eg. pluriotent cell based products • Treat/prevent disease by adding cells

ATMP cell therapies repair, regenerate or replace human tissue Non-homologous function Donor cells ATMP cure, diagnose or prevent disease Manufacture Gene modification gene therapies Tissue Engineered Product (TEP) Somatic Cell Therapy Medicinal Product (s. CTMP) ATMP/ex-vivo GTMP Outside the body What are ATMPs? ATMP/GTMP Medicine inserts ’recombinant’ gene sequences into the body for therapeutic, prophylactic or diagnostic effect. Inside the body in-vivo GTMP ’Recombinant’ (cut and paste) gene technology Every ATMP starts with either donor/patient cells and/or recombinant gene technology

Biologics Medical Devices (93/42/EEC) Medicinal Products (2001/83/EC) Type of treatment/product modality Medical Devices Tissue Therapy Cell Therapy Gene Therapy Vaccines Combined ATMP Tissue Engineered Product (TEP) Somatic cell therapy medicinal product (s. CTMP) Gene Therapy Medicinal Product (GTMP) eg. DNA vaccines or recombinant virus AGAINST infectious disease eg. Holoclar, Alofisel, lab expanded blood stem cells, MSC for arthritis eg. Kymriah, Imlygic, EV with recombinant m. RNA, ILP 100, invitro transcribed m. RNA medical device plus a TEP, s. CTMP or GTMP eg. Spherox, lab grown skin for burns treatment, PTEV (Verigraft) Advanced Therapy Medicinal Products (ATMP) (1394/2007) eg. medical device only, deceullarised scaffold eg. skin transplant for burns treatment eg. bone marrow transplant, blood transfusions Tissues and Cells (2004/23/EC), Blood (2002/98/EC) ATMPs Biotech eg. Insulin, antibodies, EV with transgenic protein By EMA legal classification all gene therapies are GTMPs Chemicals eg. Aspirin, Spinraza Synthetic oligonucleotide, legally speaking, are not gene therapies NOT ATMPs ATMP Sweden

Heather Main - Communications Mail: heather. main@sll. se Phone: 072 468 2088 Website: https: //www. atmpsweden. se/ Linked. In: https: //www. linkedin. com/company/atmpsweden/
- Slides: 5