WG 4 Standards Implementation Issues with CDISC Data













- Slides: 15

WG 4: Standards Implementation Issues with CDISC Data Models Team Update: June 18, 2012

Meeting Purpose and Outcomes Purpose Provide WG 4 volunteers with an update of WG 4’s activities since the conference , help guide them through the Wiki, Kickoff for Data Guide Outcomes • Volunteers are able to review WG 4 content on wiki and post discussion comments • Next steps for Data Guide Sub-team • Volunteers will understand their action items and WG 4’s next steps

Agenda • • • Introductions Re-cap of Conference outcomes Wiki Overview Data Guide Sub-team Kickoff Next Steps: Co-Leads and Volunteers Q&A

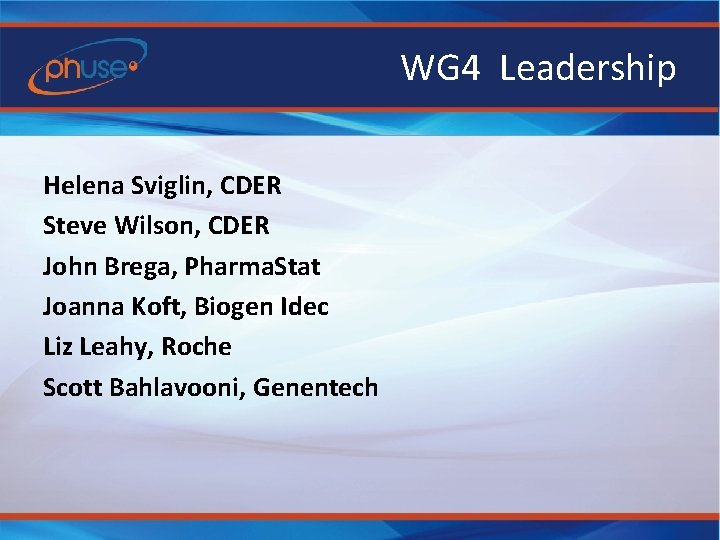
WG 4 Leadership Helena Sviglin, CDER Steve Wilson, CDER John Brega, Pharma. Stat Joanna Koft, Biogen Idec Liz Leahy, Roche Scott Bahlavooni, Genentech

WG 4 Conference Outcomes Identified 3 workstreams: 1. Data Guide 2. SDTM submission exceptions 3. Challenges Industry facing with implementing standards

Since the conference…. - Weekly Teleconference - Developed content on the wiki - Creating project-specific pages - Gathering Data Guide Examples/Analysis - Testing Wiki Functionality - Update to Steering Committee

Wiki Overview Main source of content! – General Information – Meeting Minutes and Presentations – Current Projects • Discussion Threads – Issues List: The parking lot is open!

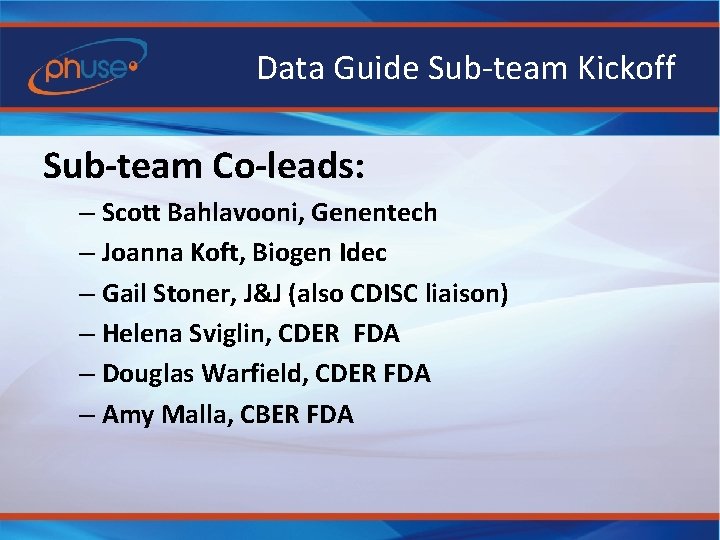
Data Guide Sub-team Kickoff Sub-team Co-leads: – Scott Bahlavooni, Genentech – Joanna Koft, Biogen Idec – Gail Stoner, J&J (also CDISC liaison) – Helena Sviglin, CDER FDA – Douglas Warfield, CDER FDA – Amy Malla, CBER FDA

Data Guide Sub-team Kickoff Project Plan: – Review existing Data Guide examples and begin discussion around content – Identify “required” and optional/recommended sections of content and develop clear descriptions – Create best practice content (review, finalize) – Repeat above process format/structure – Create proposed template for Data Guide

Data Guide Sub-team Kickoff Project Plan: – Proposal to FDA via sub-team representative – Create master Data Guide incorporating FDA feedback – Review/Finalize – Next Steps

Data Guide Sub-team Kickoff Next Steps: Co-Leads – Co-leads to post project plan with timeline to wiki – Begin to consolidate comments – Set up next teleconference update

Data Guide Sub-team Kickoff Next Steps: Volunteers – Create Phuse wiki account – Add a reply to the “Getting Familiar with the Wiki” discussion – Review Data Guide Analysis and Examples – Provide feedback on Doug Warfield’s example – Contribute to the “Data Guide Content” discussion – Add additional discussion topics as needed

Data Guide Sub-team Kickoff FDA perspective Douglas Warfield CDER/FDA

Meeting Purpose and Outcomes Purpose Provide WG 4 volunteers with an update of WG 4’s activities since the conference , help guide them through the Wiki, Kickoff for Data Guide Outcomes • Volunteers are able to review WG 4 content on wiki and post discussion comments • Next steps for Data Guide Sub-team • Volunteers will understand their action items and WG 4’s next steps

Questions