West Nile Virus 2002 Laboratory Perspectives Laboratories Branch

West Nile Virus 2002 Laboratory Perspectives Laboratories Branch MOHLTC Pauline George

Overview Test procedures WNV Statistics Laboratory Challenges
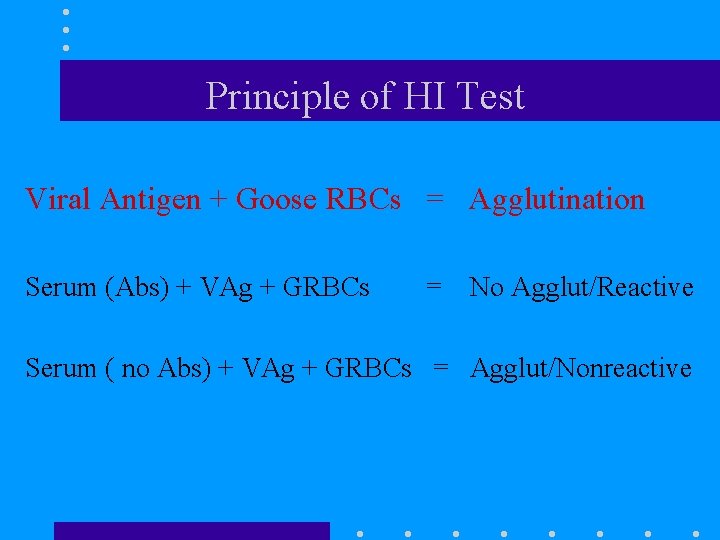
Principle of HI Test Viral Antigen + Goose RBCs = Agglutination Serum (Abs) + VAg + GRBCs = No Agglut/Reactive Serum ( no Abs) + VAg + GRBCs = Agglut/Nonreactive
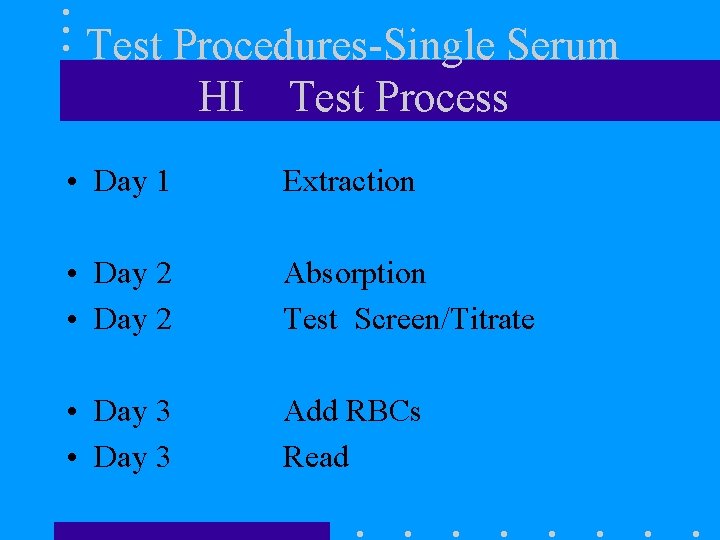
Test Procedures-Single Serum HI Test Process • Day 1 Extraction • Day 2 Absorption Test Screen/Titrate • Day 3 Add RBCs Read

Confirmatory Test Winnipeg Federal Lab • 2 week TAT • Plaque Reduction Neutralization test Testing on paired serum against likely viruses Presence/absence neutralizing antibody • PCR Tissue/CSF

WNV Tests 1999 -2002

WNV Tests May - Oct 1999 -2002

Confirmed West Nile Human Cases Ontario (to Nov 4, 2002)

Probable* West Nile Human Cases Ontario (to Nov 4, 2002)

WVN Cases Summary (to Nov 4, 2002) • • Confirmed * (PRNT) Did not confirm *(PRNT) Probable * (HI) Suspect ^ * seroconversion ^ seropositive 49 9 89 176

Laboratory Challenges 2002 • • • Substantially increased test volumes Staffing resources Reagent Availability Equipment Antigenic Variation Increased incoming/outgoing telephone calls Other routine tests Training TDG Space Allocation

Future Challenges 2002 and Beyond • • • Funding Staffing In-house tests vs Commercial Tests Availability? Case criteria Data Analysis Training Confirmatory Test Availability Antigen consistency Antigen source/cost?

Future Challenges 2002 and Beyond cont’d • For confirmatory testing in Ontario • Requires Level 3 Biosafety lab • PRNT confirmatory assay • PCR • Culture

Acknowledgements OPHL Arboviruses & Rickettsiae Laboratory Staff Dr. M. Fearon, I Guglielmi, E Cheung, Canadian Science Centre for Human & Animal Health , Winnipeg Viral Zoonotic Staff Dr. M. Drebot
- Slides: 14