Welcome Warmup Please open the Socrative app on









![Ionic Bond Li + Li+ F F - [He] 1 s 1 s 2 Ionic Bond Li + Li+ F F - [He] 1 s 1 s 2](https://slidetodoc.com/presentation_image_h2/aecf551abc04a38f0da996f3f3f1e5ae/image-18.jpg)


























































- Slides: 127

Welcome! • Warm-up Please open the Socrative app on your phone and answer the two MC questions to the best of your ability (do not discuss it yet).

Reminders • 15 school days to Winter Break • 34 school days to End of 1 st Semester [January 28 th ]

Until Then… What you can expect from me: -Clear delineation of assignments And due dates. -Availability before and after school What I need from you: -Sensitivity to other classes (proper notice) -Attentiveness to directions -Clarifying questions when material is unclear -Assignments turned in on time, complete *Incomplete work will be handed back *Work which ignores directions will be handed back

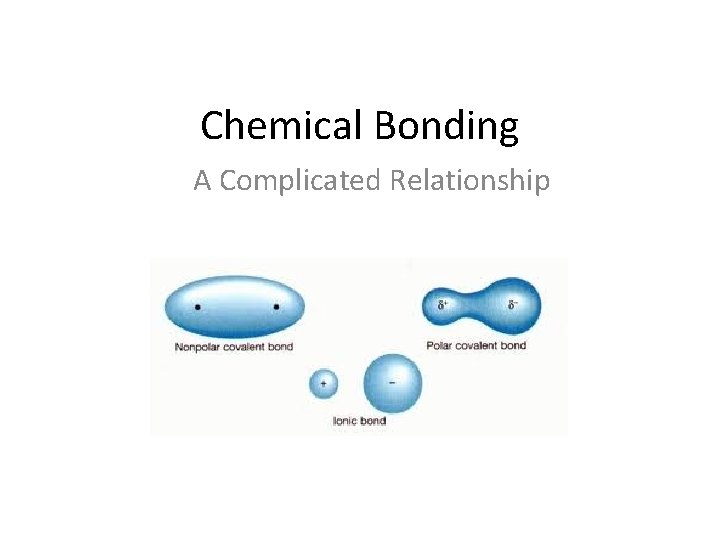
Chemical Bonding A Complicated Relationship

Today • • The Tools for Understanding Bonding Valence Electrons Lewis Dot Diagrams The Octet Rule Oxidation States

Why do we care? Ionic Bonds

Why do we care? Covalent Bonds

Some of the tools we already have

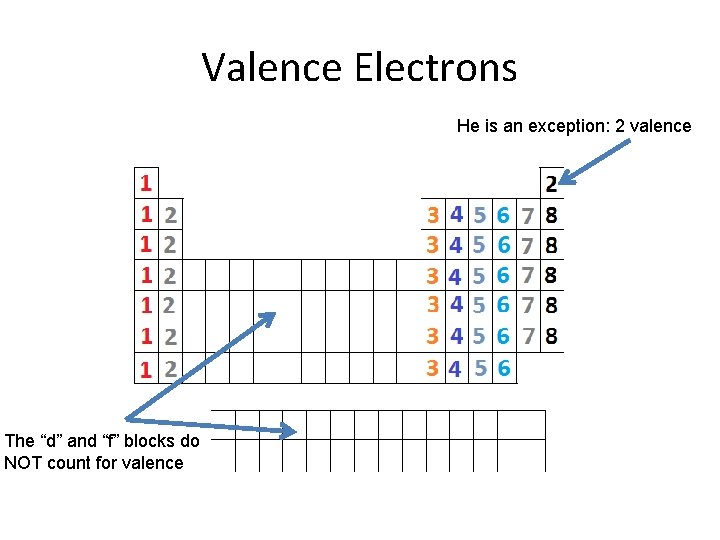
Valence Electrons He is an exception: 2 valence The “d” and “f” blocks do NOT count for valence

Lewis Dot Structures • Simplified way of showing only the highest energy (valence) electrons. • Will only ever have a maximum of 8 dots • All elements in the same group (column) will have the same Lewis dot diagram.

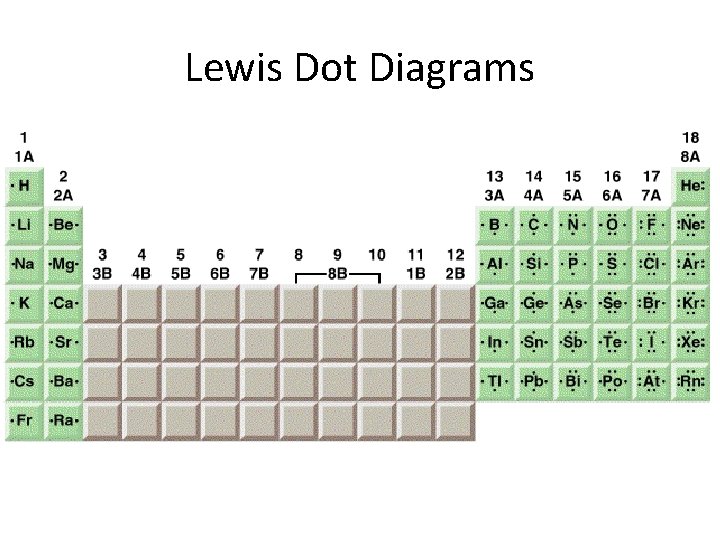
Lewis Dot Diagrams

Some things we still need to learn

The Octet Rule • Electron Configuration is related to stability! Ne… 1 s 2 2 p 6 F… 1 s 2 2 p 5 • Noble Gases have 8 e- in their highest E level, so they are perfectly happy to not react. • Everything else is trying to reach a more stable state, so electrons must either be donated or accepted in order to complete an octet How many bonds will “F” form?

Octet Rule • Bottom Line: Elements tend to gain or lose electrons in order to achieve the electron configuration of a noble gas (i. e. a full valence shell!) Exceptions: Hydrogen (H) and Helium (He) Because both are in the first E level (n=1) which only has 2 valence


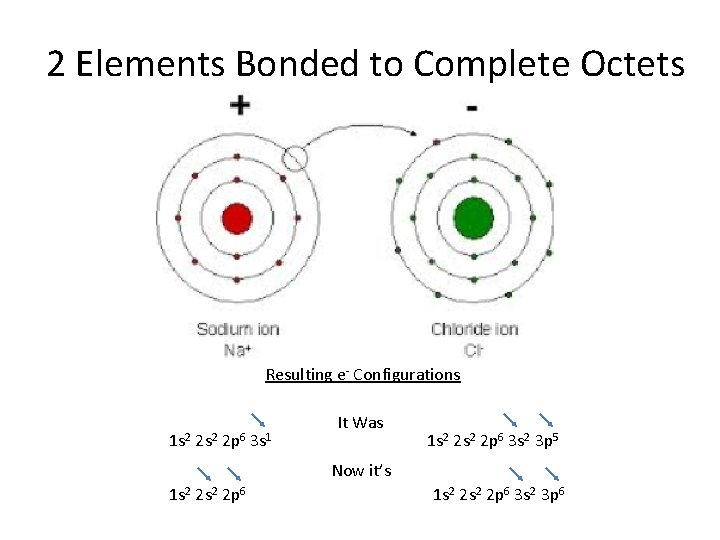
2 Elements Bonded to Complete Octets Resulting e- Configurations 1 s 2 2 p 6 3 s 1 It Was 1 s 2 2 p 6 3 s 2 3 p 5 Now it’s 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6

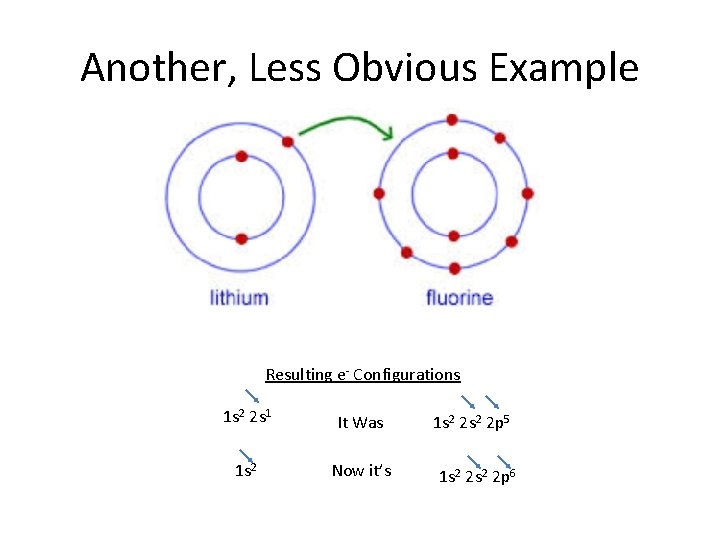
Another, Less Obvious Example Resulting e- Configurations 1 s 2 2 s 1 It Was 1 s 2 Now it’s 1 s 2 2 p 5 1 s 2 2 p 6
![Ionic Bond Li Li F F He 1 s 1 s 2 Ionic Bond Li + Li+ F F - [He] 1 s 1 s 2](https://slidetodoc.com/presentation_image_h2/aecf551abc04a38f0da996f3f3f1e5ae/image-18.jpg)
Ionic Bond Li + Li+ F F - [He] 1 s 1 s 2 2[Ne] 2 s 22 p 6 1 s 22 s 1 1 s 22 p 5 Li+ Li 1 s 2 s 1 s 2 p + + F 1 s 2 s 2 p 18

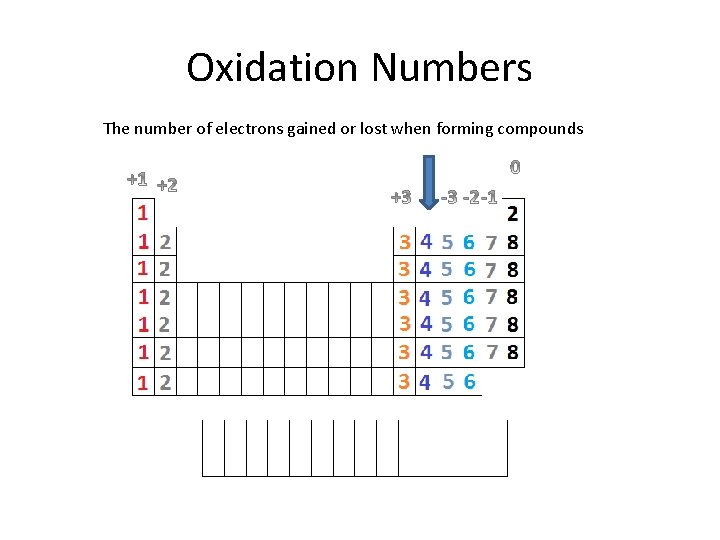
Oxidation Numbers The number of electrons gained or lost when forming compounds

Practice How many valence electrons does Beryllium (Be) have? 2 What does the dot diagram of Beryllium (Be) look like? How many electrons would Beryllium (Be) need to gain to have a full valence? 6 How many electrons would Beryllium (Be) need to lose to have a full valence? 2 What is the likely oxidation number of Beryllium (Be)? 2+

Practice How many valence electrons does Phosphorus (P) have? 5 What does the dot diagram of Phosphorus (P) look like? How many electrons would Phosphorus (P) need to gain to have a full valence? 3 How many electrons would Phosphorus (P) need to lose to have a full valence? 5 What is the likely oxidation number of phosphorus (P)? 3 -

IF YOU CANNOT DETERMINE THE OXIDATION NUMBER, YOU WILL NOT UNDERSTAND OR BE ABLE TO COMPLETE IONIC BONDING FORMULAS!

Check Yourself! Before you wreck yourself On a separate sheet of paper: 1) Write the oxidations number for N, Se, I, Cs, Ba, and Ca 2) We did not talk about group 4 oxidation numbers. List a possibility and justify it in one sentence

Tomorrow Ionic Bonds!

Warm-Up 12/1 How many valence electrons does bromine (Br) have? 7 What does the dot diagram of bromine (Br) look like? How many electrons would bromine (Br) need to gain to have a full valence? 1 How many electrons would bromine (Br) need to lose to have a full valence? 7 What is the likely oxidation number of bromine (Br)? 1 -

Yesterday • • The Tools for Understanding Bonding Valence Electrons Lewis Dot Diagrams The Octet Rule Oxidation States

Today • Understand ionic bonds by constructing and writing proper formulas and compound names.

Ionic Bonding Activity • In groups of 2 to 3, you will receive a packet of ion cards. • Using the numbers on the board, count your cards and make sure you have the right number of each. • Follow the directions in the packet and answer the questions in detail (ask Mr. White for help). • The packet should be completed in class.

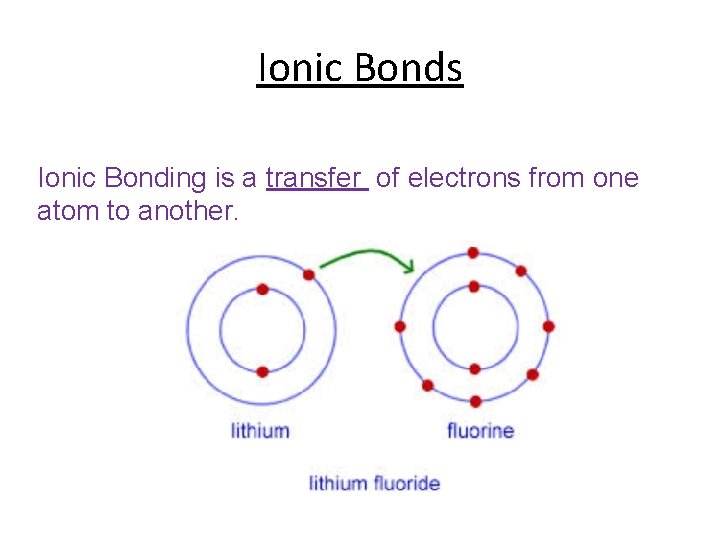
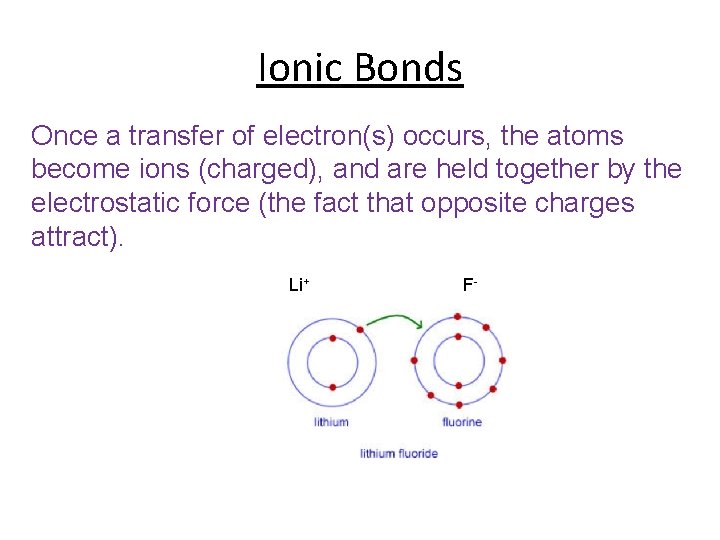
Ionic Bonds Ionic Bonding is a transfer of electrons from one atom to another.

Ionic Bonds Once a transfer of electron(s) occurs, the atoms become ions (charged), and are held together by the electrostatic force (the fact that opposite charges attract). Li+ F-

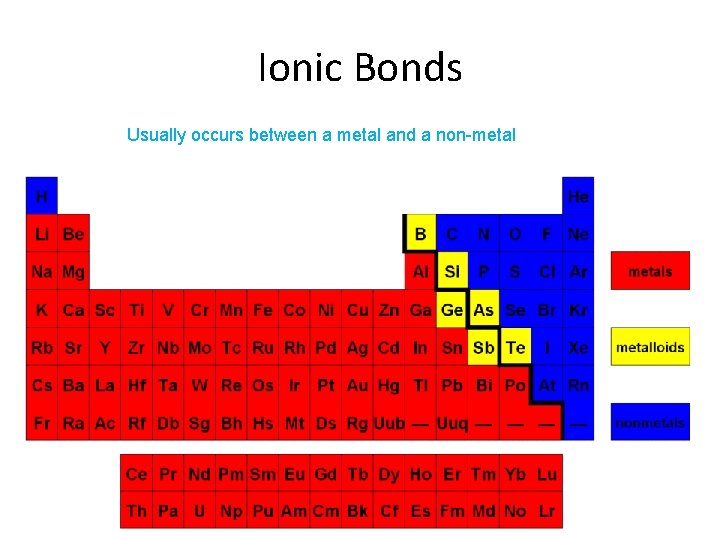
Ionic Bonds Usually occurs between a metal and a non-metal

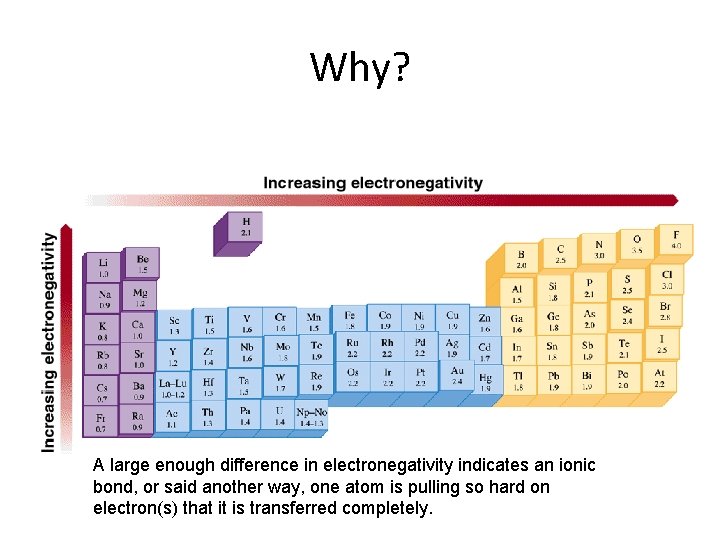
Why? A large enough difference in electronegativity indicates an ionic bond, or said another way, one atom is pulling so hard on electron(s) that it is transferred completely.

Bottom Line Bond types are classified by the difference in electronegativity of the atoms involved

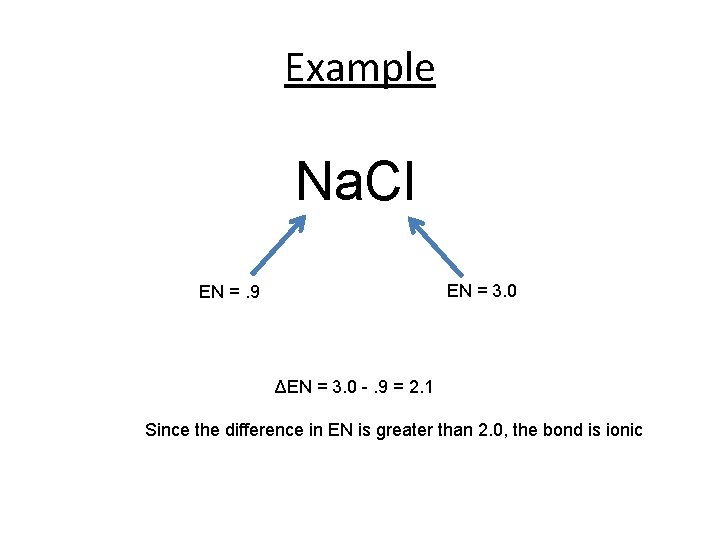
Example Na. Cl EN = 3. 0 EN =. 9 ΔEN = 3. 0 -. 9 = 2. 1 Since the difference in EN is greater than 2. 0, the bond is ionic

Ionic Compounds 1) Made up of cations (+ charged) and anions (- charged) 2) Correctly written compounds are electrically neutral (no overall charge)

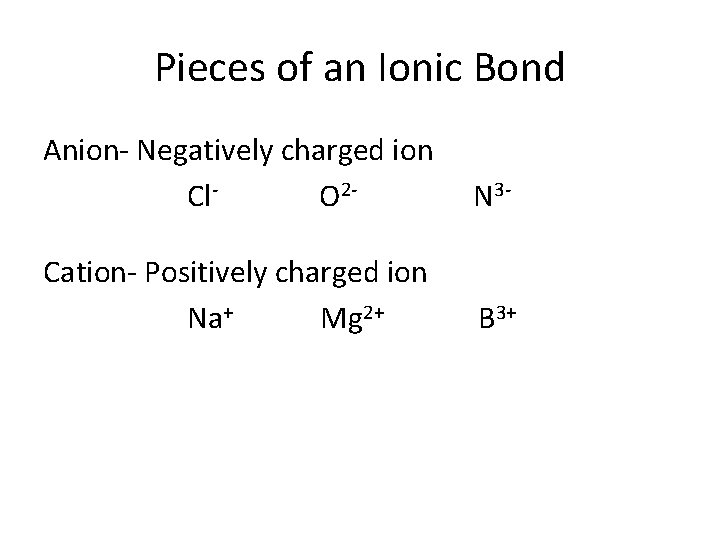
Pieces of an Ionic Bond Anion- Negatively charged ion Cl. O 2 - N 3 - Cation- Positively charged ion Na+ Mg 2+ B 3+

Monatomic Ions • Have the same name as the elemental form • Exist as only atoms of a single element • First component in an ionic compound

Polyatomic Ions • Consist of atoms of more than one element • Come as a “package deal” when forming ionic compounds • Don’t get mixed up between the net charge of the ion and the individual charges

Formulas for Binary Compounds ➢ Contain a monatomic cation (metal) and a monatomic anion (nonmetal) ➢ Metal comes first ➢ Non-metal comes second ➢ The overall charge must be 0 ➢ You can use subscipts (i. e. O 2) to get the overall charge to zero. ➢ DO NOT WRITE THE CHARGE IN THE COMPOUND

Thursday 12/3 • Please have out your worksheet (pink) from yesterday • You will need: – Notes – P. Table – Polyatomic Reference Sheet (from yesterday)

Today Rules for Systematically Naming Ionic Compounds: • Binary Compounds (Single cation, single anion) • Binary Compounds with “d” block elements (using roman numerals) • Polyatomic Compounds (with and without “d” block elements)

Rules Naming Binary Ionic Compounds Li 2 O • List the name of the cation first, and keep the name the way it is.

Rules Naming Binary Ionic Compounds Li 2 O • List the name of the cation first, and keep the name the way it is. Name: Lithium ………. .

Rules Naming Binary Ionic Compounds Li 2 O • List the name of the cation first, and keep the name the way it is. Name: Lithium ………. . • List the name of the anion second, change the ending to “ide” if it is simply an element.

Rules Naming Binary Ionic Compounds Li 2 O • List the name of the cation first, and keep the name the way it is. Name: Lithium ………. . • List the name of the anion second, change the ending to “ide” if it is simply an element. Name: Lithium Oxide

Rules Naming Binary Ionic Compounds Li 2 O Full Name: Lithium Oxide

Examples with “d” block elements (transition metals) d block

Rules Naming Binary Ionic Compounds Fe. Cl 2 • List the name of the cation first, and keep the name the way it is.

Rules Naming Binary Ionic Compounds Fe. Cl 2 • List the name of the cation first, and keep the name the way it is.

Rules Naming Binary Ionic Compounds Fe. Cl 2 • List the name of the cation first, and keep the name the way it is. Name: Iron

Rules Naming Binary Ionic Compounds Fe. Cl 2 • List the name of the cation first, and keep the name the way it is. Name: Iron ( ) ……… • This time, however, leave a set of empty parentheses that we will later come back to fill in the oxidation state with.

Rules Naming Binary Ionic Compounds Fe. Cl 2 • List the name of the cation first, and keep the name the way it is. Name: Iron ( ) chloride • This time, however, leave a set of empty parentheses that we will later come back to fill in the oxidation state with. • Name the anion second and change the ending to “ide” if it is simply an element.

Rules Naming Binary Ionic Compounds • Fe. Cl 2 To complete this name, we need to determine the oxidation state of the iron. Name: Iron ( ) chloride

Rules Naming Binary Ionic Compounds • Fe. Cl 2 To complete this name, we need to determine the oxidation state of the iron. Name: Iron ( ) chloride • To do this, we figure out the total charge of the anion.

Rules Naming Binary Ionic Compounds • Fe. Cl 2 To complete this name, we need to determine the oxidation state of the iron. Name: Iron ( ) chloride • To do this, we figure out the total charge of the anion. Cl has a 1 - oxidation, and there are two of them

Rules Naming Binary Ionic Compounds • Fe. Cl 2 To complete this name, we need to determine the oxidation state of the iron. Name: Iron ( II ) chloride • To do this, we figure out the total charge of the anion. Cl has a 1 - oxidation, and there are two of them So… we need a 2+ oxidation on the Fe to cancel out Cl 2

Rules Naming Binary Ionic Compounds Fe. Cl 2 Full Name: Iron ( II ) chloride

Examples with polyatomic ions

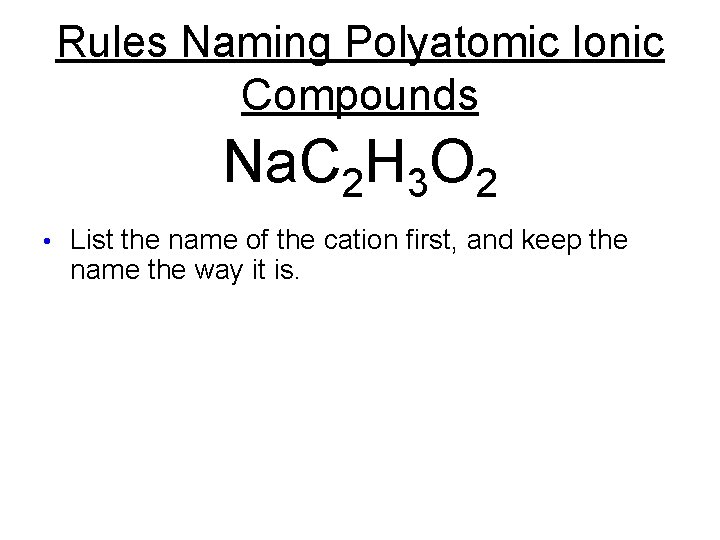
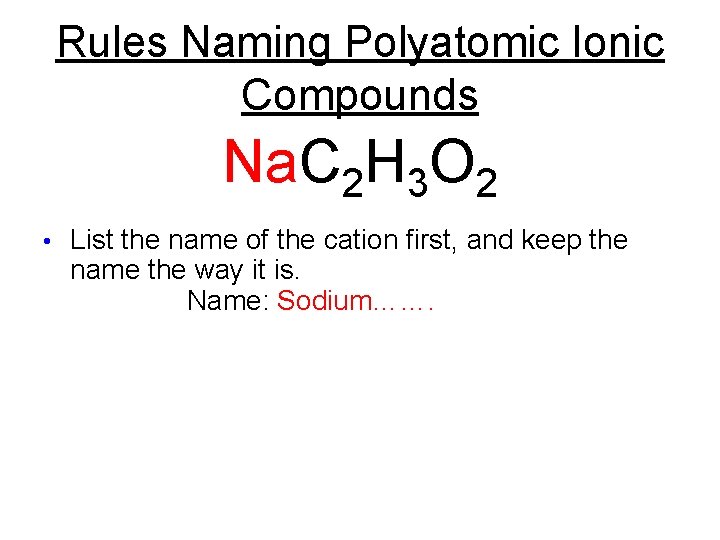
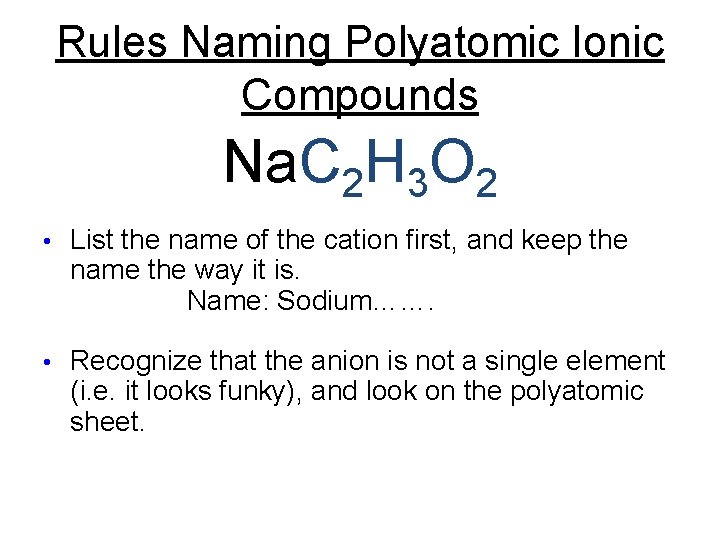
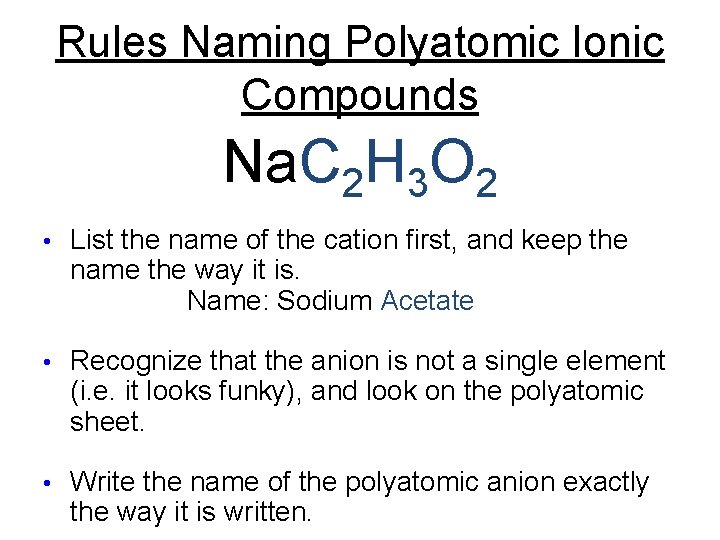
Rules Naming Polyatomic Ionic Compounds Na. C 2 H 3 O 2 • List the name of the cation first, and keep the name the way it is.

Rules Naming Polyatomic Ionic Compounds Na. C 2 H 3 O 2 • List the name of the cation first, and keep the name the way it is. Name: Sodium…….

Rules Naming Polyatomic Ionic Compounds Na. C 2 H 3 O 2 • List the name of the cation first, and keep the name the way it is. Name: Sodium……. • Recognize that the anion is not a single element (i. e. it looks funky), and look on the polyatomic sheet.

Rules Naming Polyatomic Ionic Compounds Na. C 2 H 3 O 2 • List the name of the cation first, and keep the name the way it is. Name: Sodium Acetate • Recognize that the anion is not a single element (i. e. it looks funky), and look on the polyatomic sheet. • Write the name of the polyatomic anion exactly the way it is written.

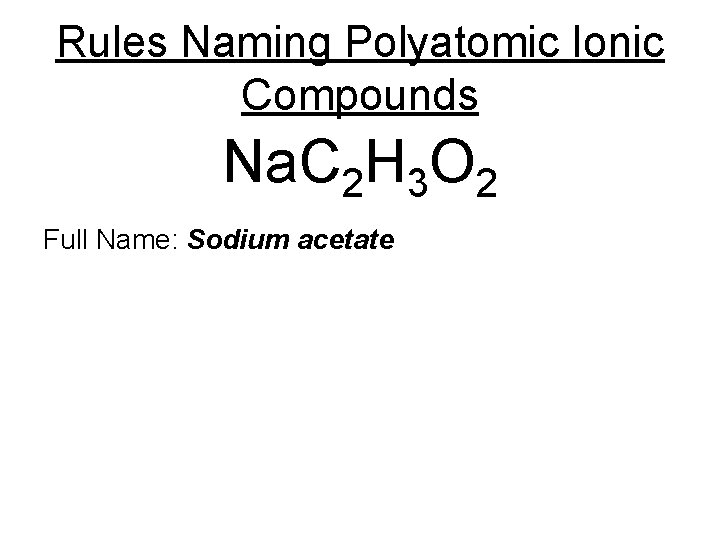
Rules Naming Polyatomic Ionic Compounds Na. C 2 H 3 O 2 Full Name: Sodium acetate

Check Yourself 1) Write the name for the ionic compound which forms from: Li. F Cd. CO 3 Fe(NO 3)2

Hang on to your green and pink worksheet for now.

Monday 12/7 • Put your name on a blank sheet of paper and label it neatly from 1 -11 (Leave 3 spaces between each number).

Ionic Compound Characteristics • At the counters, you will find 11 ionic salts: • For each salt, describe the compound’s color and form (ex: crystals, powder, pellets, flakes, etc. ). • If the name of the compound is given, give the formula; if the formula of the compound is given, give the name. Ex: Na. Cl description: white crystals name: sodium chloride Ex. sodium chloride description: white crystals formula: Na. Cl

Bonding Thus Far • Ionic Bonding is the transfer of electrons between two atoms. • Atoms in an ionic bond are held together through electrostatic interaction, not electron sharing. • An ionic bond is made up of a cation (+ charge) and an anion (- charge) • Atoms connected by ionic bonds are called ionic compounds

Natural World



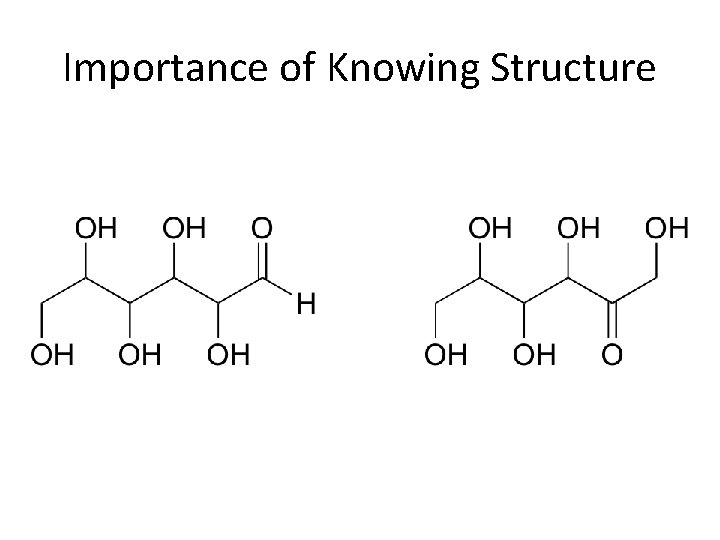
Covalent Bonding • Result from the sharing of a pair of electrons between atoms • Atoms connected by covalent bonds are called molecules • Molecules can have the same molecular formula, but different structural formulas

Importance of Knowing Structure

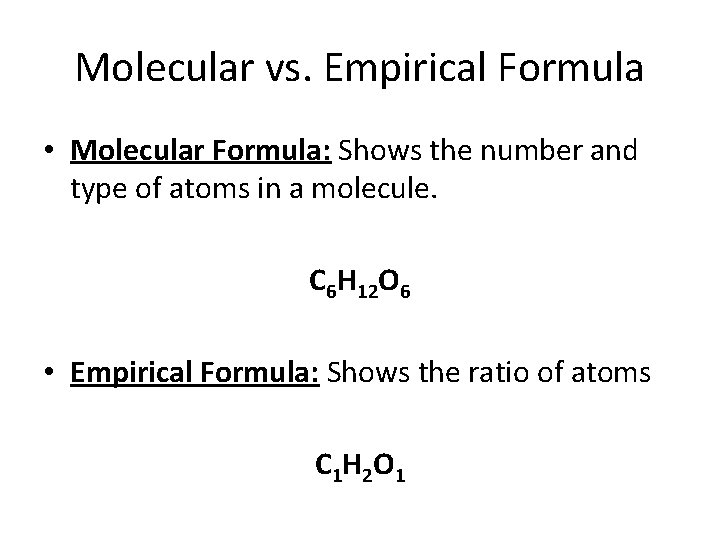
Molecular vs. Empirical Formula • Molecular Formula: Shows the number and type of atoms in a molecule. C 6 H 12 O 6 • Empirical Formula: Shows the ratio of atoms C 1 H 2 O 1

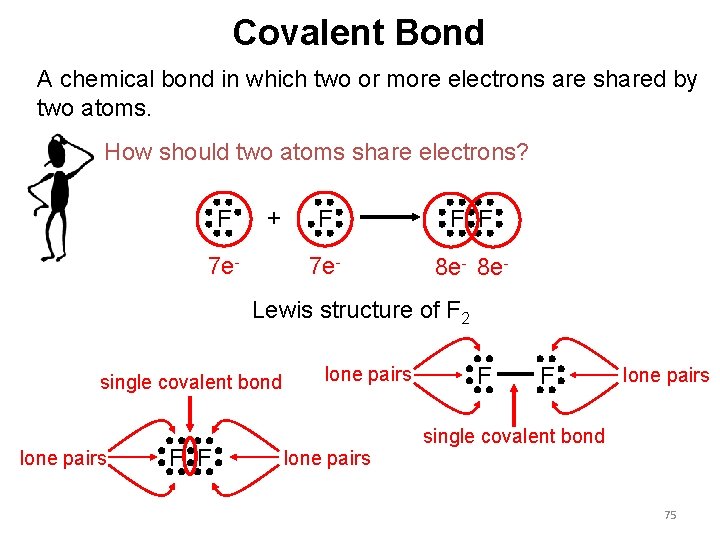
Covalent Bond A chemical bond in which two or more electrons are shared by two atoms. How should two atoms share electrons? F + 7 e- F F F 7 e- 8 e- Lewis structure of F 2 single covalent bond lone pairs F F lone pairs single covalent bond 75

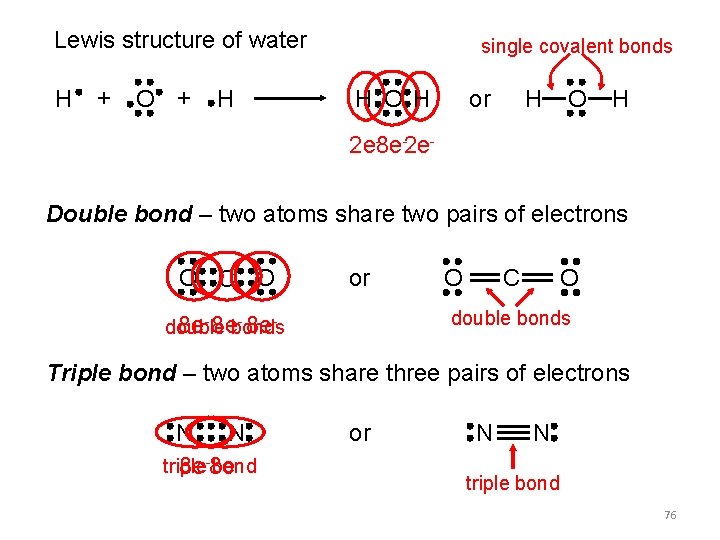
Lewis structure of water H + O + H single covalent bonds H O H or H O H 2 e-8 e-2 e. Double bond – two atoms share two pairs of electrons O C O or O O C double bonds - 8 e 8 e- 8 ebonds double Triple bond – two atoms share three pairs of electrons N N triple bond 8 e-8 e or N N triple bond 76

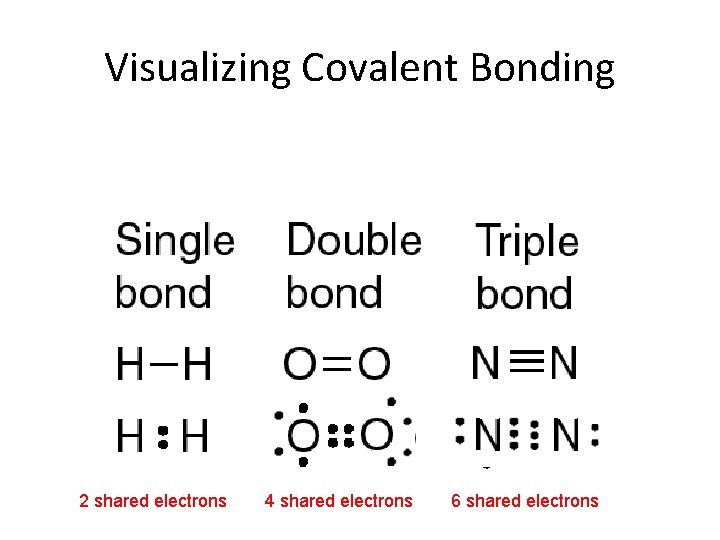
Visualizing Covalent Bonding 2 shared electrons 4 shared electrons 6 shared electrons

NAMING

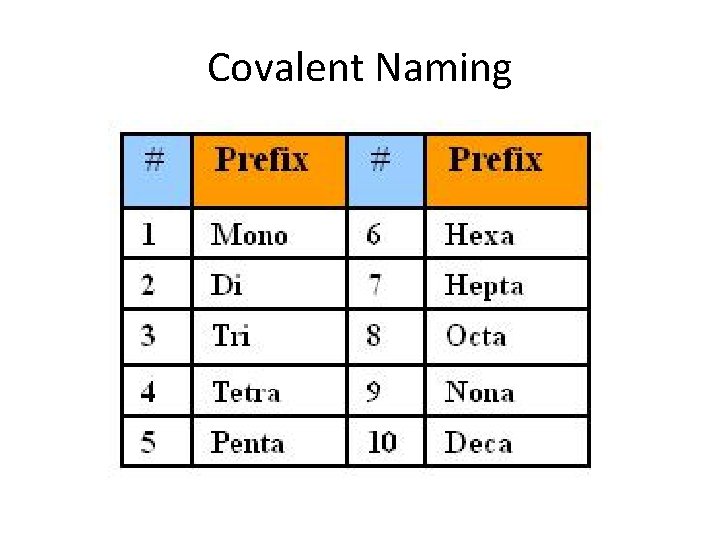
Covalent Naming • Numerical prefixes are used to denote number of atoms • No prefix for only a single atom • “-ide” is added to the end of the more electronegative element

Common Names • Some compounds have common names

H 2 O- Water

NH 3 - Ammonia

CHCl 3 - Chloroform

Covalent Naming

• Please turn in your blue covalent naming worksheet.

DRAWING STRUCTURES

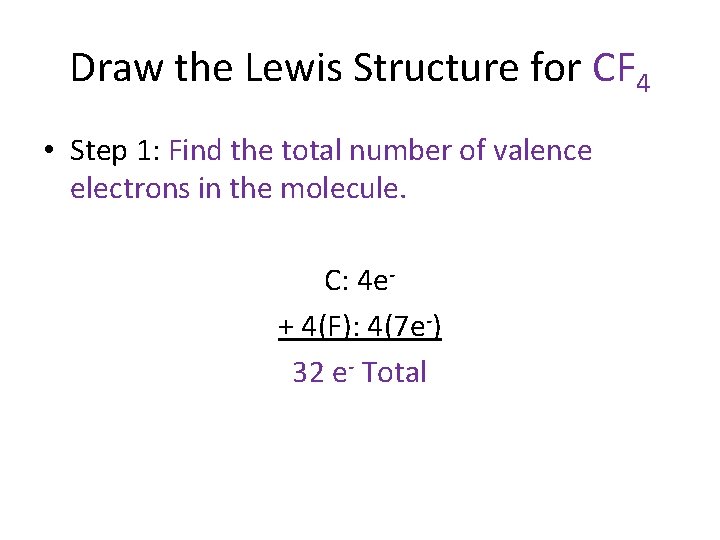
Draw the Lewis Structure for CF 4 • Step 1: Find the total number of valence electrons in the molecule. C: 4 e+ 4(F): 4(7 e-) 32 e- Total

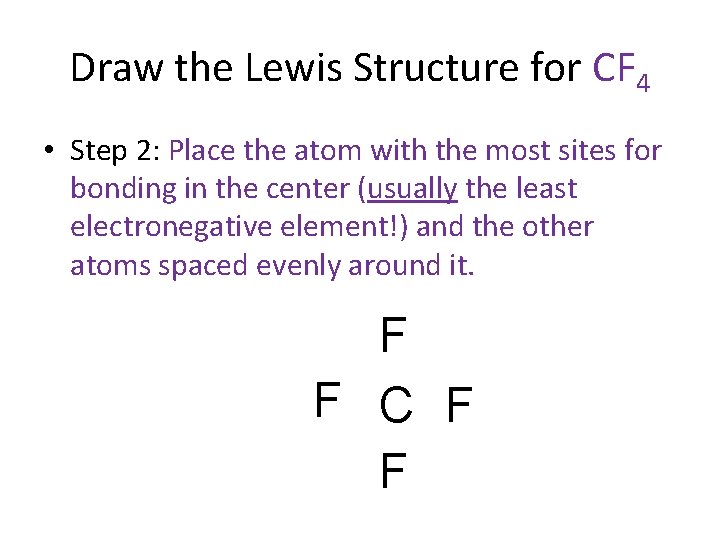
Draw the Lewis Structure for CF 4 • Step 2: Place the atom with the most sites for bonding in the center (usually the least electronegative element!) and the other atoms spaced evenly around it. F F C F F

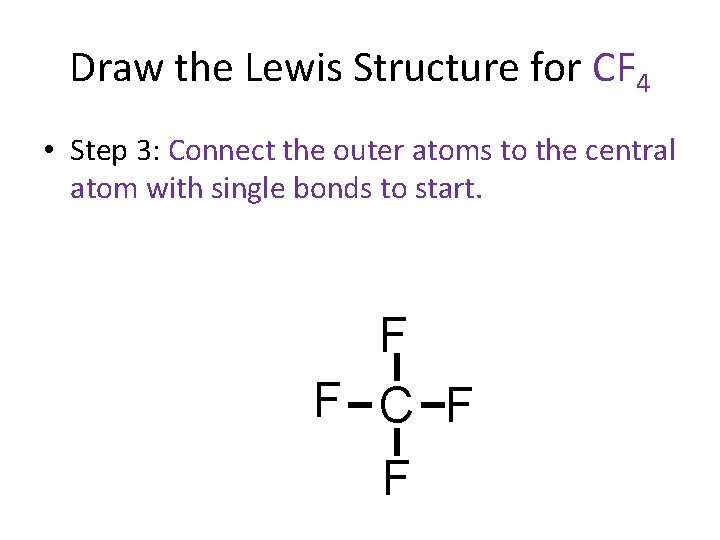
Draw the Lewis Structure for CF 4 • Step 3: Connect the outer atoms to the central atom with single bonds to start. F F C F F

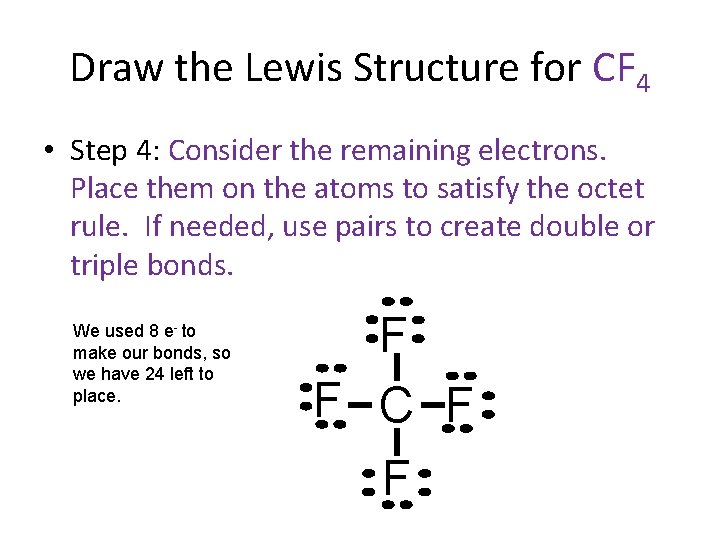
Draw the Lewis Structure for CF 4 • Step 4: Consider the remaining electrons. Place them on the atoms to satisfy the octet rule. If needed, use pairs to create double or triple bonds. We used 8 e- to make our bonds, so we have 24 left to place. F F C F F

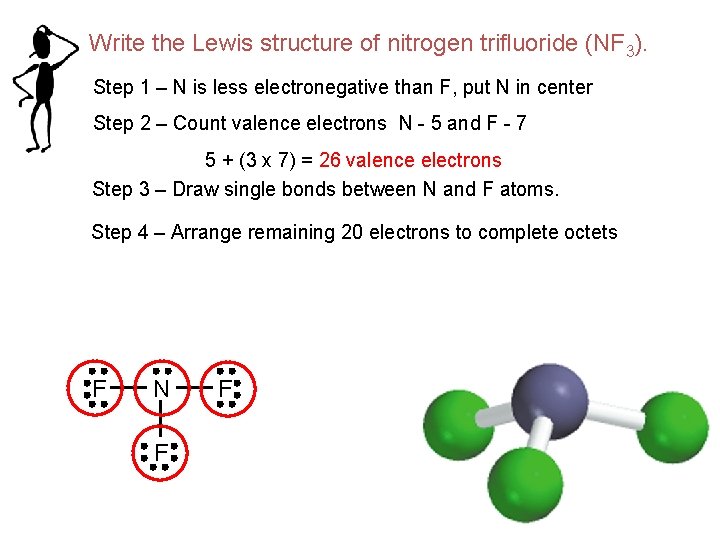
Write the Lewis structure of nitrogen trifluoride (NF 3). Step 1 – N is less electronegative than F, put N in center Step 2 – Count valence electrons N - 5 and F - 7 5 + (3 x 7) = 26 valence electrons Step 3 – Draw single bonds between N and F atoms. Step 4 – Arrange remaining 20 electrons to complete octets F N F F 91

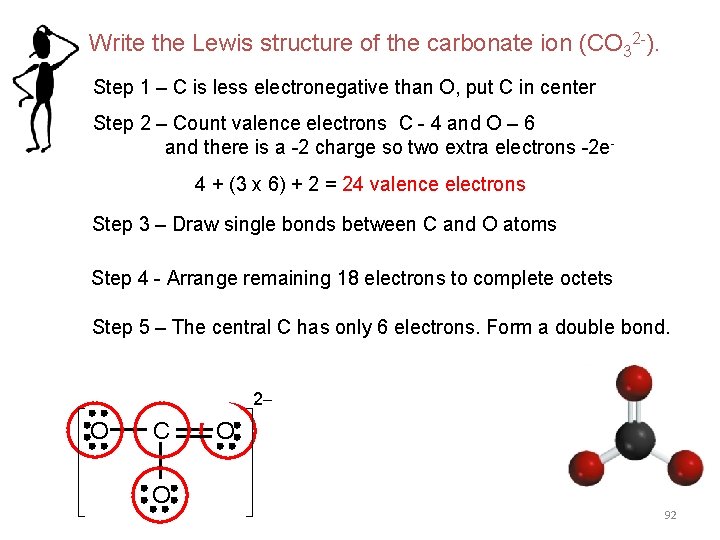
Write the Lewis structure of the carbonate ion (CO 32 -). Step 1 – C is less electronegative than O, put C in center Step 2 – Count valence electrons C - 4 and O – 6 and there is a -2 charge so two extra electrons -2 e 4 + (3 x 6) + 2 = 24 valence electrons Step 3 – Draw single bonds between C and O atoms Step 4 - Arrange remaining 18 electrons to complete octets Step 5 – The central C has only 6 electrons. Form a double bond. 2 - O C O O 92

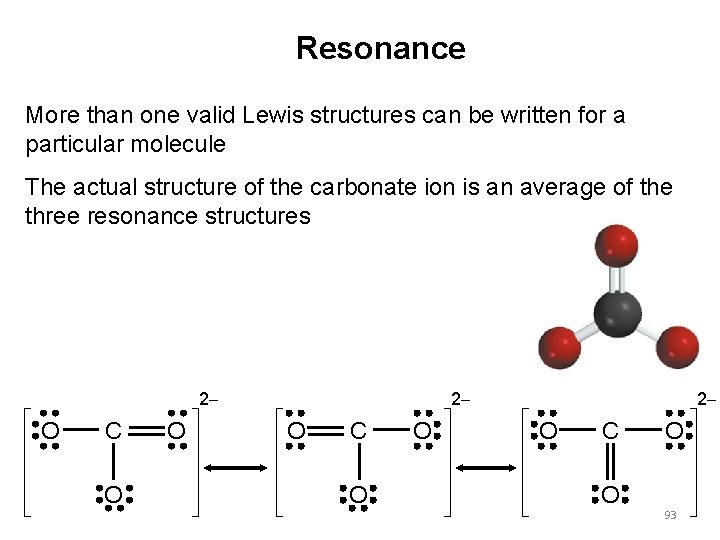
Resonance More than one valid Lewis structures can be written for a particular molecule The actual structure of the carbonate ion is an average of the three resonance structures - 2 - O C O O - - 2 - O C O O 93 -

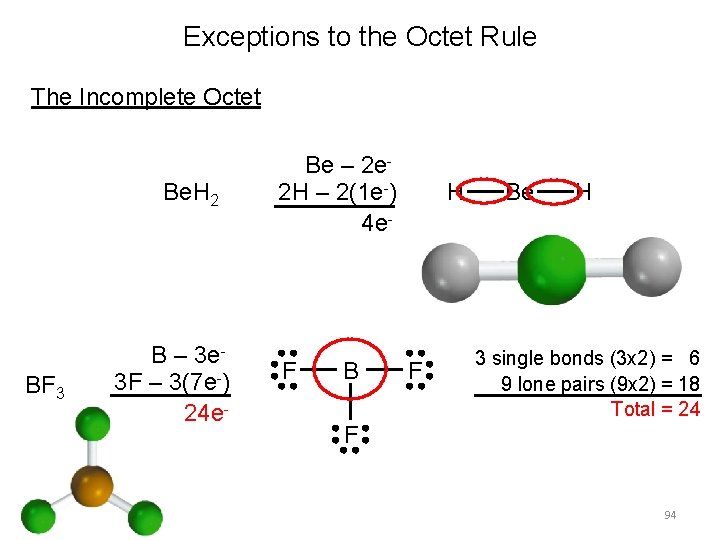
Exceptions to the Octet Rule The Incomplete Octet Be. H 2 BF 3 B – 3 e 3 F – 3(7 e-) 24 e- Be – 2 e 2 H – 2(1 e-) 4 e- F B H F Be H 3 single bonds (3 x 2) = 6 9 lone pairs (9 x 2) = 18 Total = 24 F 94

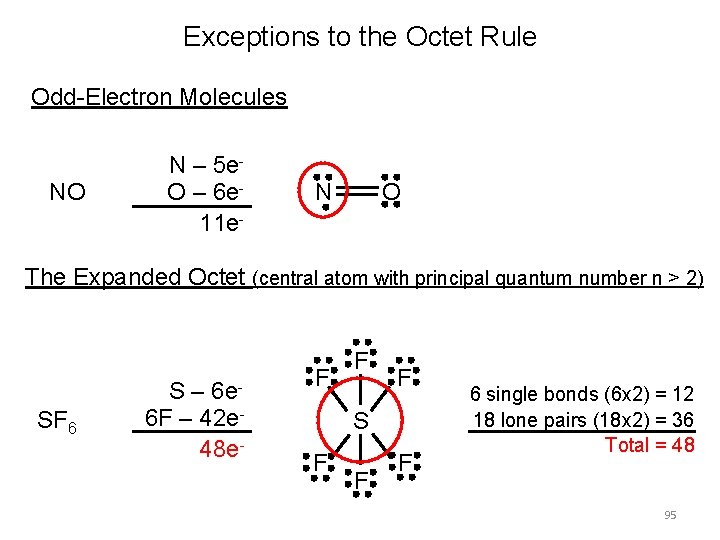
Exceptions to the Octet Rule Odd-Electron Molecules NO N – 5 e. O – 6 e 11 e- N O The Expanded Octet (central atom with principal quantum number n > 2) SF 6 S – 6 e 6 F – 42 e 48 e- F F F S F F F 6 single bonds (6 x 2) = 12 18 lone pairs (18 x 2) = 36 Total = 48 95

But Mr. White… Why? ? ?

MAGIC!

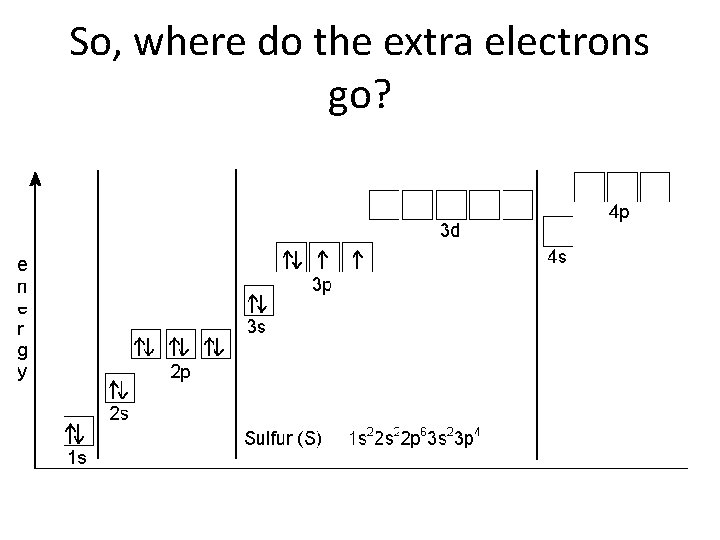
So, where do the extra electrons go?

BOND POLARITY

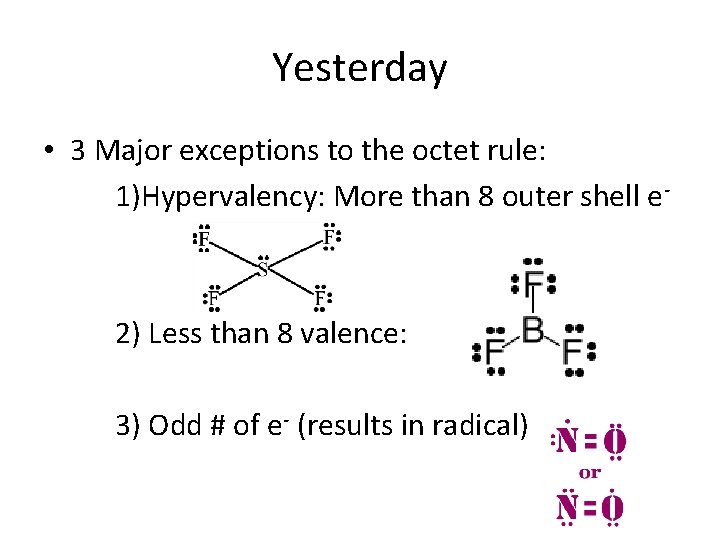
Yesterday • 3 Major exceptions to the octet rule: 1)Hypervalency: More than 8 outer shell e- 2) Less than 8 valence: 3) Odd # of e- (results in radical)

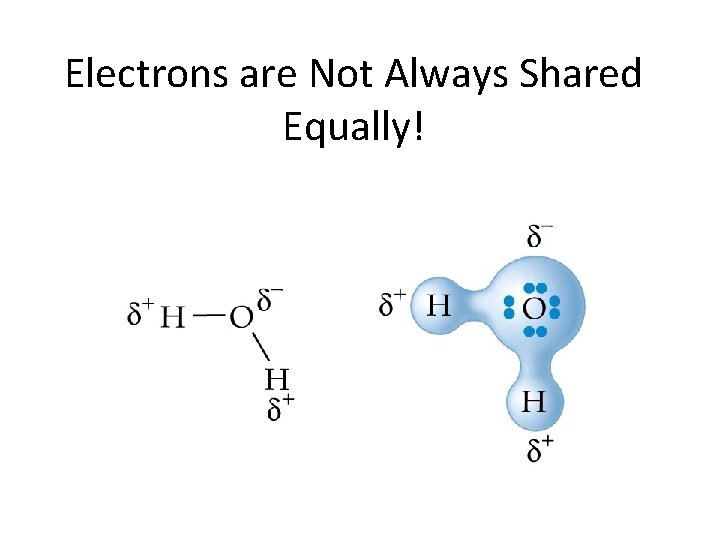
Electrons are Not Always Shared Equally!

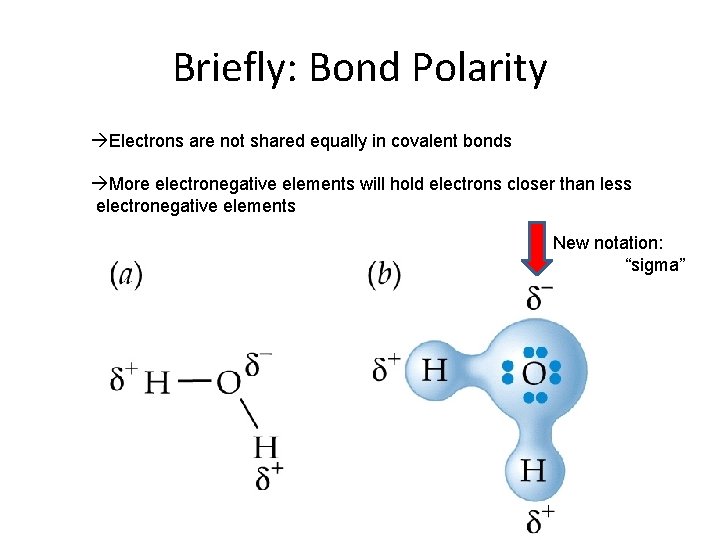
Briefly: Bond Polarity àElectrons are not shared equally in covalent bonds àMore electronegative elements will hold electrons closer than less electronegative elements New notation: “sigma”

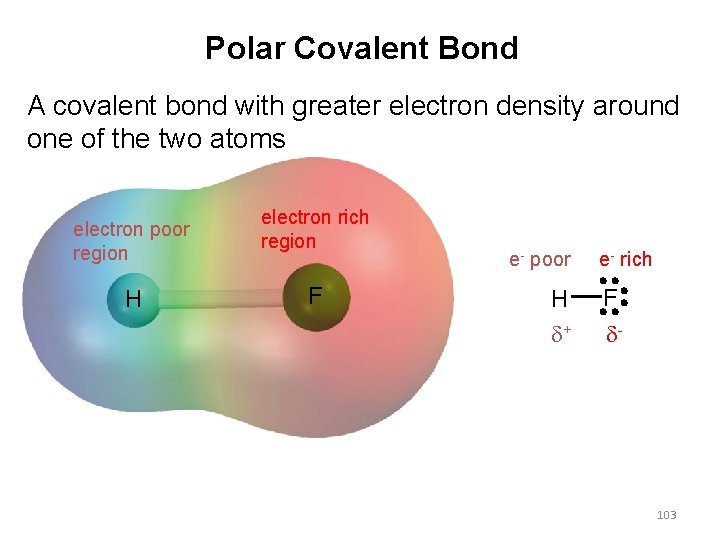
Polar Covalent Bond A covalent bond with greater electron density around one of the two atoms electron poor region H electron rich region F e- poor H d+ e- rich F d- 103

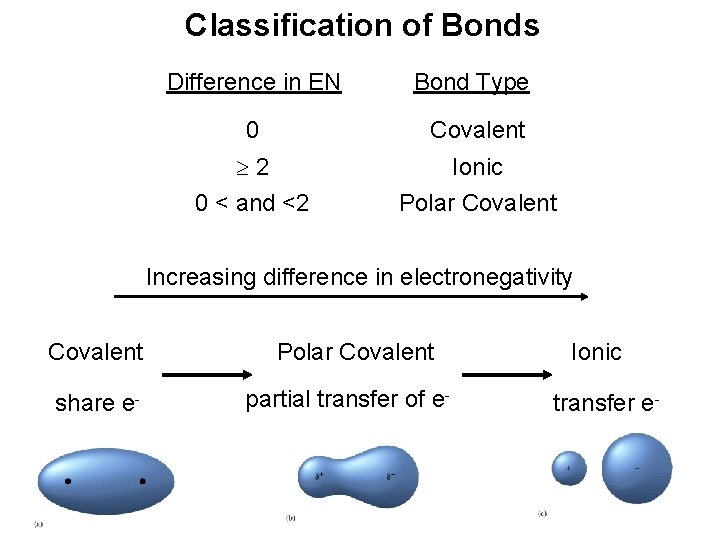
Classification of Bonds Difference in EN Bond Type 0 Covalent 2 0 < and <2 Ionic Polar Covalent Increasing difference in electronegativity Covalent share e- Polar Covalent partial transfer of e- Ionic transfer e- 104

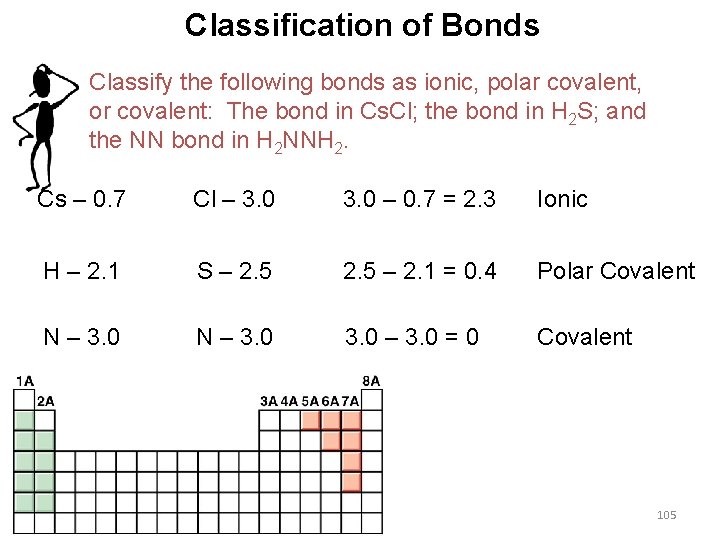
Classification of Bonds Classify the following bonds as ionic, polar covalent, or covalent: The bond in Cs. Cl; the bond in H 2 S; and the NN bond in H 2 NNH 2. Cs – 0. 7 Cl – 3. 0 – 0. 7 = 2. 3 Ionic H – 2. 1 S – 2. 5 – 2. 1 = 0. 4 Polar Covalent N – 3. 0 – 3. 0 = 0 Covalent 105

Covalent Bond Lengths Bond Type Bond Length (pm) C -C 154 C C 133 C C 120 C -N 143 C N 138 C N 116 Bond Lengths Triple bond < Double Bond < Single Bond 106

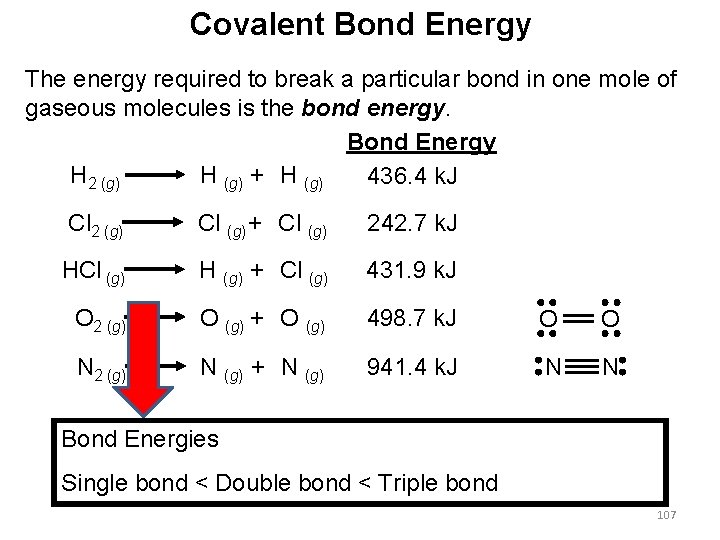
Covalent Bond Energy The energy required to break a particular bond in one mole of gaseous molecules is the bond energy. Bond Energy H 2 (g) H (g) + H (g) 436. 4 k. J Cl 2 (g) Cl (g) + Cl (g) 242. 7 k. J HCl (g) H (g) + Cl (g) 431. 9 k. J O 2 (g) O (g) + O (g) 498. 7 k. J O O N 2 (g) N (g) + N (g) 941. 4 k. J N N Bond Energies Single bond < Double bond < Triple bond 107

Practice • PH 3 • N 2 O 3 • CO • HI

Check Yourself • Write the chemical name for the following compounds according to the rules for either ionic or covalent naming: Fe. SO 3

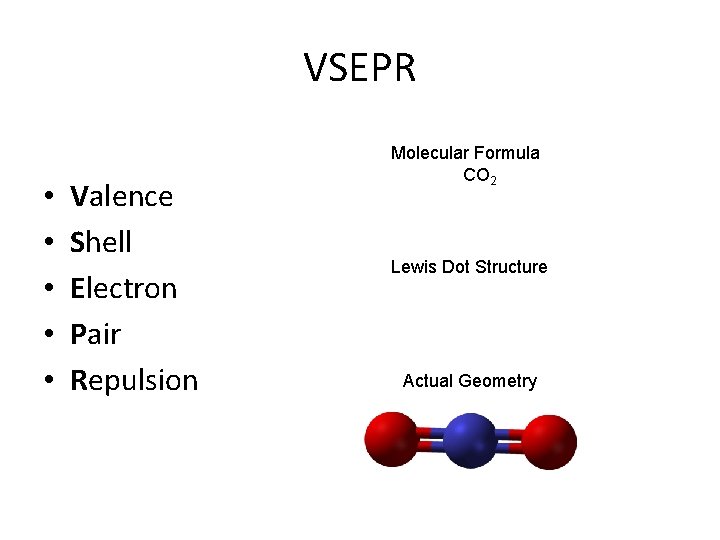
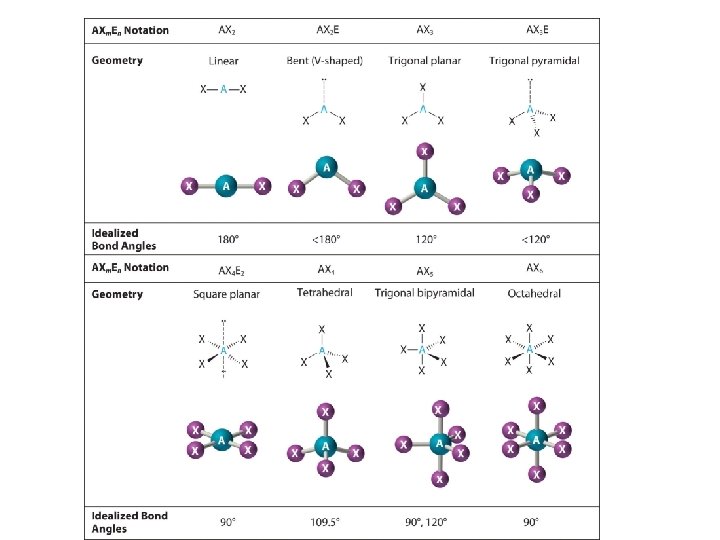
VSEPR • • • Valence Shell Electron Pair Repulsion Molecular Formula CO 2 Lewis Dot Structure Actual Geometry

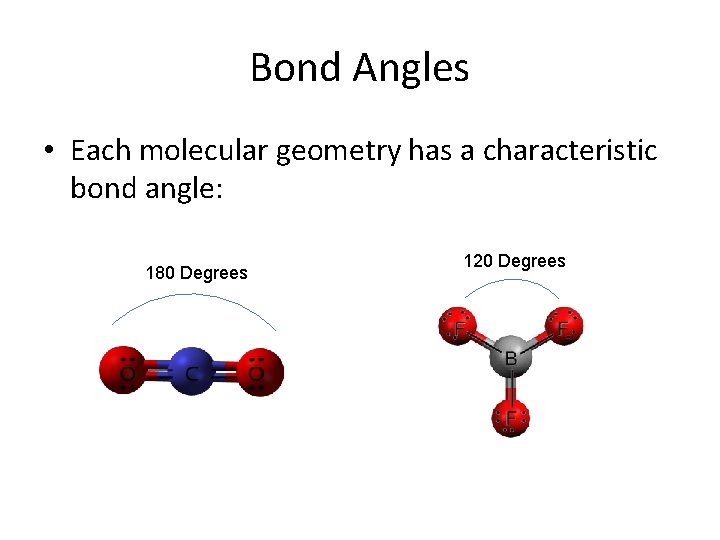
Bond Angles • Each molecular geometry has a characteristic bond angle: 180 Degrees 120 Degrees

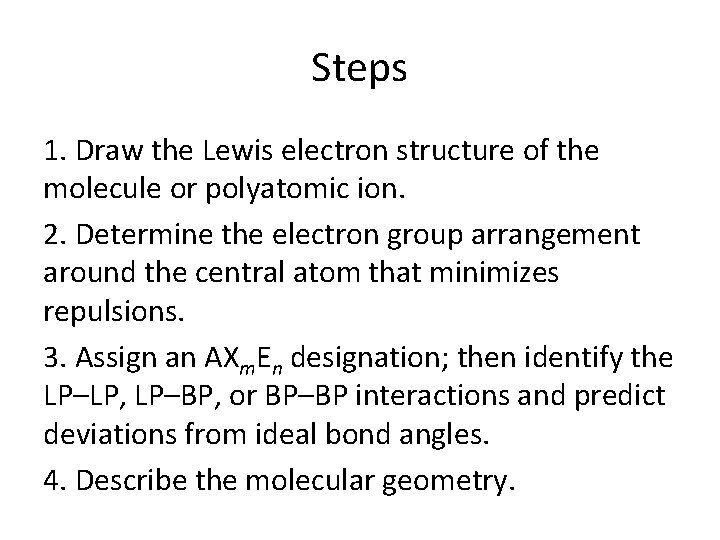
Steps 1. Draw the Lewis electron structure of the molecule or polyatomic ion. 2. Determine the electron group arrangement around the central atom that minimizes repulsions. 3. Assign an AXm. En designation; then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations from ideal bond angles. 4. Describe the molecular geometry.


Geometry Lab Activity • You may work in pairs • Draw the Lewis structure before constructing the model • Careful not to lose pieces

What about lots of bonds?

Rules • Each person only has 1, 2, or 3 valence electrons • Must form single bonds before forming double or triple bonds • NO OFF-TASK BEHAVIOR

Complex Molecules Ex: Proteins!

Geometry Lab Activity • You may work in pairs • Draw the Lewis structure before constructing the model • Careful not to lose pieces


Linear

Bent

Trigonal Planar

Tetrahedral

Pyramidal

Bond Length

Molecular Polarity

TOMORROW! • Finish Modeling • Discuss molecular polarity • Pass out review sheet