Welcome to Quarter 2 Make sure you have























![Examples 1. Write the shorthand electron configuration of Mn. [Ar]4 s 23 d 5 Examples 1. Write the shorthand electron configuration of Mn. [Ar]4 s 23 d 5](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-36.jpg)



![Exceptions 1. Cu • not [Ar]4 s 23 d 9 but [Ar]4 s 13 Exceptions 1. Cu • not [Ar]4 s 23 d 9 but [Ar]4 s 13](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-40.jpg)
![Exceptions 4. Cr [Ar]4 s 13 d 5 5. Mo [Kr]5 s 14 d Exceptions 4. Cr [Ar]4 s 13 d 5 5. Mo [Kr]5 s 14 d](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-41.jpg)


![Examples • • • Pb: [Xe]6 s 24 f 145 d 106 p 2 Examples • • • Pb: [Xe]6 s 24 f 145 d 106 p 2](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-44.jpg)
![Transition Metals • • • Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]3 Transition Metals • • • Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]3](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-45.jpg)
![Transition Metals • • Mn: [Ar]4 s 23 d 5 Mn 2+: [Ar]3 d Transition Metals • • Mn: [Ar]4 s 23 d 5 Mn 2+: [Ar]3 d](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-46.jpg)






- Slides: 55

Welcome to Quarter 2! • Make sure you have an electron note packet, your reference packet, and a calculator • Remember ALL makeup work is due today at 3: 00 pm – NO EXCEPTIONS

Electrons in Atoms

Electrons • Scientists pursue an understanding of how electrons are arranged within atoms • Electron arrangement plays a role in chemical behavior • Early 1900 s- scientists observed that certain elements emit visible light when heated in a flame

Wave Nature of Light • Electromagnetic Radiation- form of energy that exhibits wavelike behavior as it travels through space

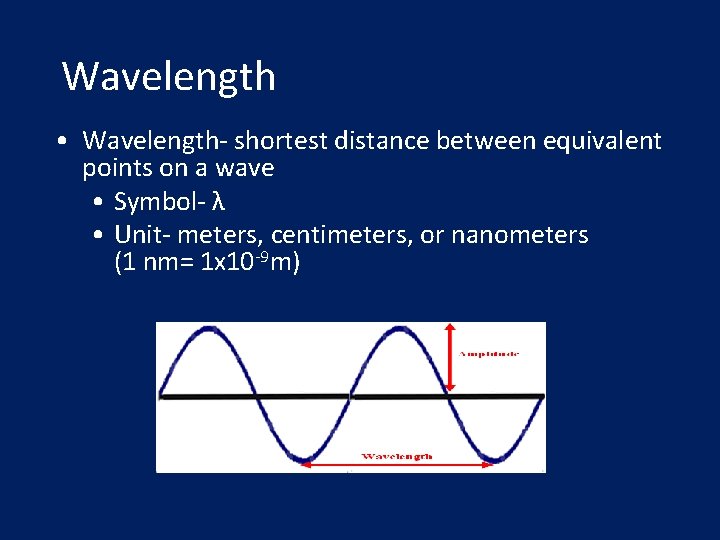
Wavelength • Wavelength- shortest distance between equivalent points on a wave • Symbol- λ • Unit- meters, centimeters, or nanometers (1 nm= 1 x 10 -9 m)

Frequency • Frequency- the number of waves that pass a given point per second • Symbol- ν • Unit- Hertz (SI Unit)= (1/s)= (s-1) cycle per second

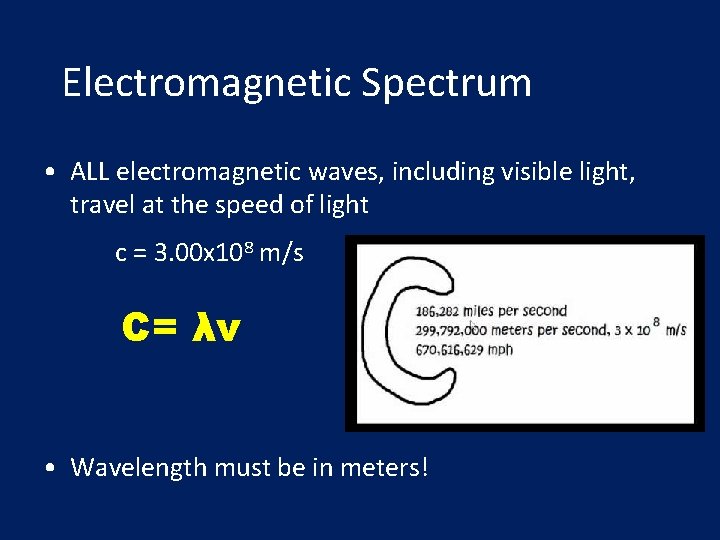
Electromagnetic Spectrum • ALL electromagnetic waves, including visible light, travel at the speed of light c = 3. 00 x 108 m/s C= λν • Wavelength must be in meters!

Electromagnetic Spectrum • Encompasses all forms of electromagnetic radiation • The only differences in the types of radiation being their wavelengths and frequencies

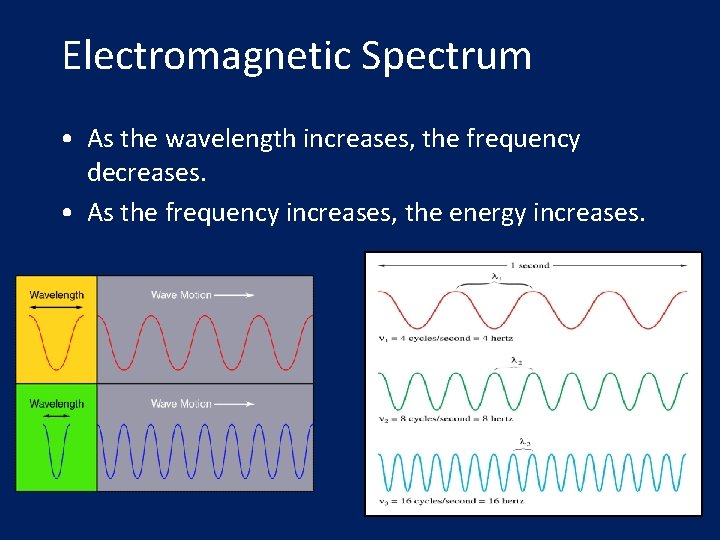
Electromagnetic Spectrum • As the wavelength increases, the frequency decreases. • As the frequency increases, the energy increases.

Calculations 1. Microwaves are used to transmit information. What is the wavelength of a microwave having a frequency of 3. 44 x 109 Hz? • C= λν • C= 3. 00 x 108 m/s • ν = 3. 44 x 109 Hz • λ = ? ? ?

3. 00 x 108 = λ(3. 44 x 109 Hz) λ= 8. 72 x 10 -2 m

2. Yellow light has a wavelength of 589 nm. What is the frequency? 5. 09 x 1014 Hz

Particle Nature of Light (Honors) • Quantum Concept • Explained why colors of heated matter correspond to different frequencies and wavelengths • Max Plank- “matter can gain or lose only in small, specific amounts called quanta” • Quantum- the minimum amount of energy that can be gained or lost by an atom

Particle Nature of Light (Honors) • Energy of a quantum is related to the frequency of the emitted radiation by the equation: Equantum= hv • E = energy • h = Plank’s Constant (6. 63 x 10 -34 J s) • v = frequency • Joule (J)= SI unit for energy

Particle Nature of Light (Honors) • Photon- a particle of EM radiation with no mass that carries a quantum of energy Ephoton= hv

Example • Calculate the quantum of energy that an object can absorb from light with a wavelength of 477 nm. 4. 17 x 10 -19 J

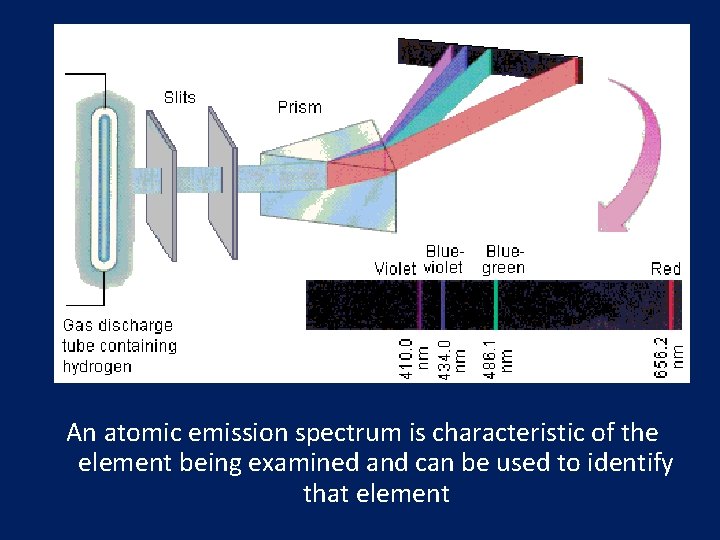
Atomic Emission Spectra • Set of frequencies of the electromagnetic waves emitted by atoms of the element • Example- The light of neon sign is produced by passing electricity through a tube filled with neon gas. Neon atoms release energy by emitting light.

An atomic emission spectrum is characteristic of the element being examined and can be used to identify that element

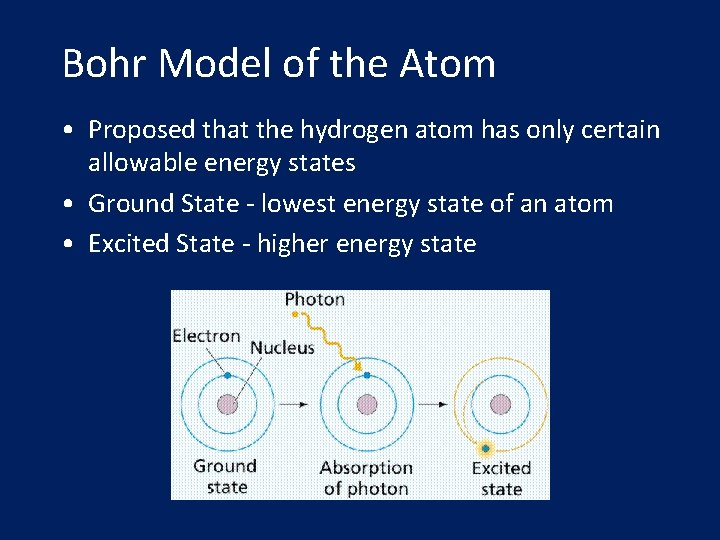
Bohr Model of the Atom • Proposed that the hydrogen atom has only certain allowable energy states • Ground State - lowest energy state of an atom • Excited State - higher energy state

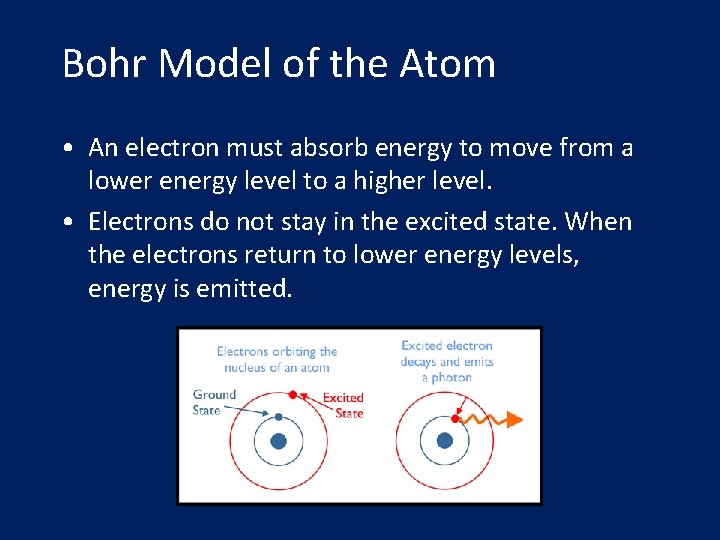
Bohr Model of the Atom • An electron must absorb energy to move from a lower energy level to a higher level. • Electrons do not stay in the excited state. When the electrons return to lower energy levels, energy is emitted.

Heisenberg Uncertainty Principle • The Heisenberg Uncertainty Principle - states that it is impossible to know precisely both the velocity and position of a particle at the same time

The Bohr Model 1. Using the Bohr Model from your packet, what is the wavelength of energy that is emitted when an electron falls from n= 6 to n=3? wavelength = 1094 nm

B) What is the frequency of this radiation? 2. 75 x 1014 Hz C) What is the energy of a photon of this radiation? (Honors) 1. 82 x 10 -19 J

• Atomic Orbital- a 3 D region around the nucleus describing the electron’s probable location

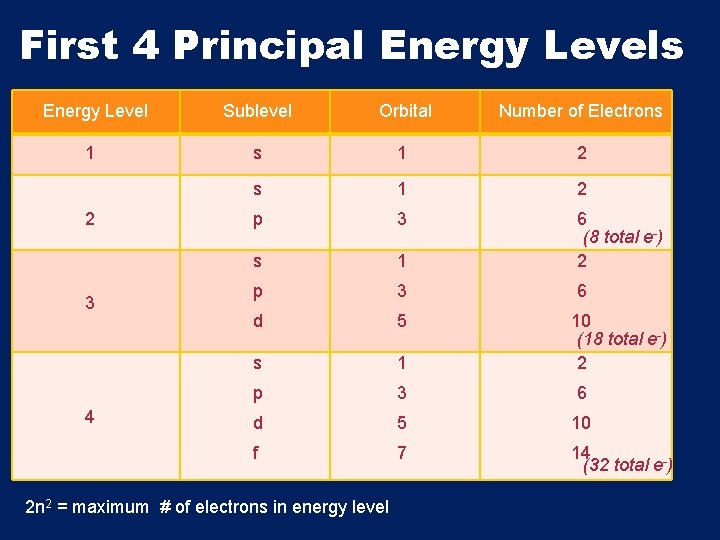
Atomic Orbitals • Energy Levels (n)- the major energy levels of an atom Ex: n = 1 energy level closest to the nucleus • Energy level → sublevel → orbital • Every orbital can hold up to 2 e-

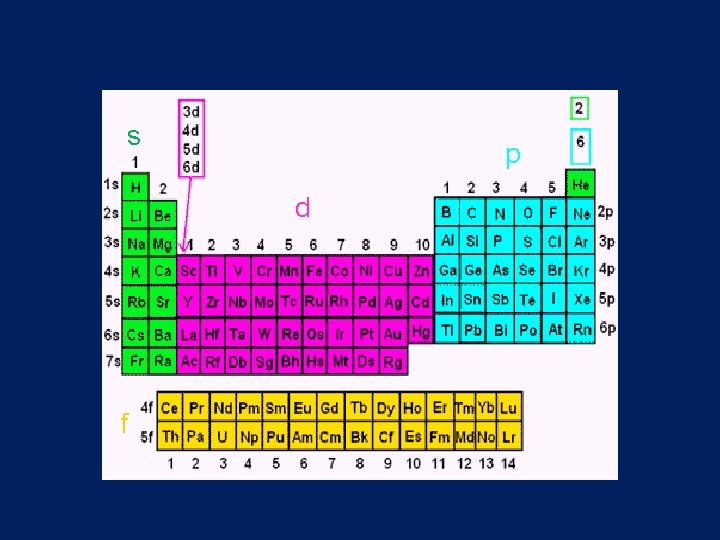
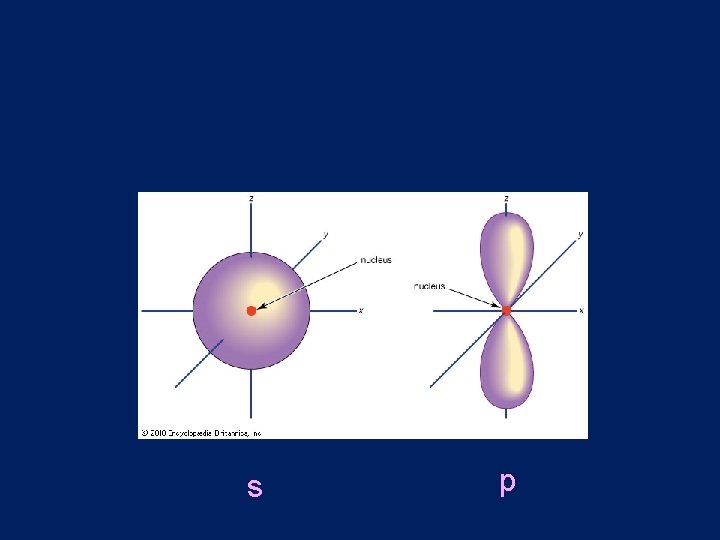
• Sublevels are represented by the letters s, p, d, f lowest energy highest energy

First 4 Principal Energy Levels Energy Level 1 2 3 4 Sublevel Orbital Number of Electrons s 1 2 p 3 s 1 6 (8 total e-) 2 p 3 6 d 5 s 1 p 3 6 d 5 10 f 7 14 (32 total e-) 2 n 2 = maximum # of electrons in energy level 10 (18 total e-) 2

Electron Arrangement in Atoms • Electron Configurations- the arrangement of electrons in an atom

Aufbau Principle : Electrons enter orbitals from lowest to highest energy

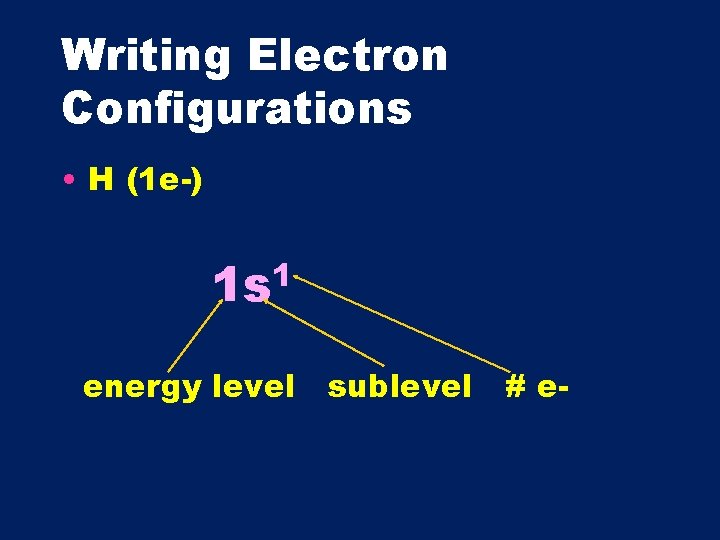
Writing Electron Configurations • H (1 e-) 1 1 s energy level sublevel # e-

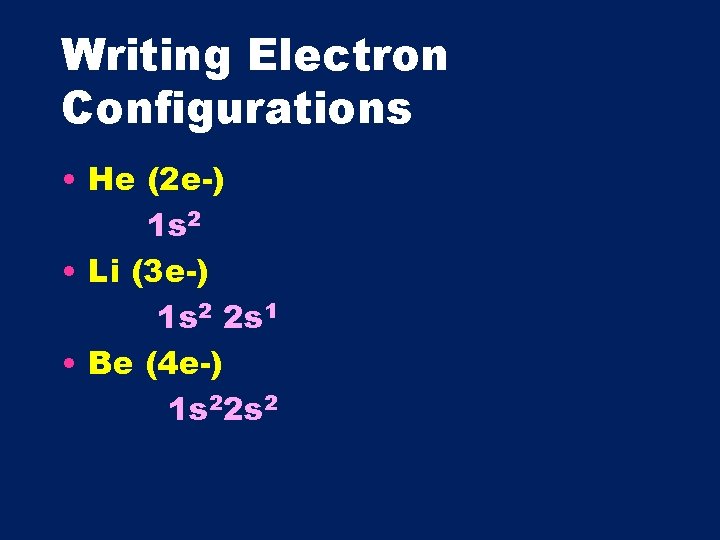
Writing Electron Configurations • He (2 e-) 1 s 2 • Li (3 e-) 1 s 2 2 s 1 • Be (4 e-) 1 s 22 s 2

Writing Electron Configurations • B (5 e-) 1 s 22 p 1 • C (6 e-) 1 s 22 p 2 • Ne (10 e-) 1 s 22 p 6

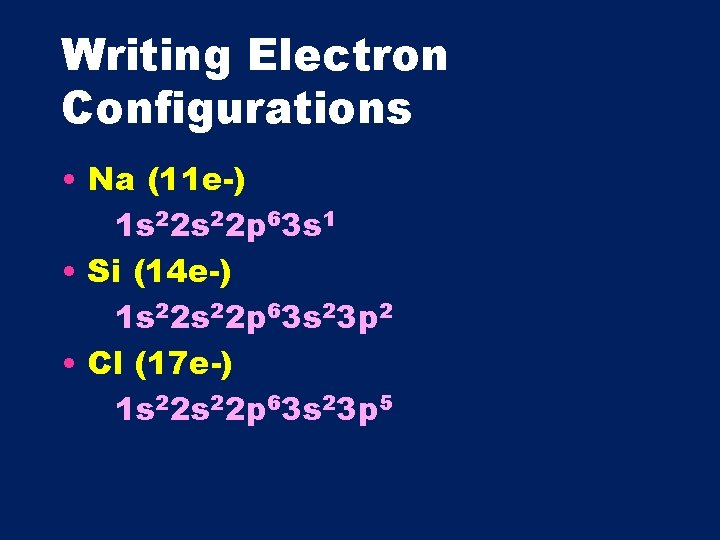
Writing Electron Configurations • Na (11 e-) 1 s 22 p 63 s 1 • Si (14 e-) 1 s 22 p 63 s 23 p 2 • Cl (17 e-) 1 s 22 p 63 s 23 p 5

s p d f

Noble Gas Configuration • Used to shorten electron configurations • Sodium: #11 - instead of 1 s 22 p 63 s 1 can be shortened to [Ne] 3 s 1
![Examples 1 Write the shorthand electron configuration of Mn Ar4 s 23 d 5 Examples 1. Write the shorthand electron configuration of Mn. [Ar]4 s 23 d 5](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-36.jpg)
Examples 1. Write the shorthand electron configuration of Mn. [Ar]4 s 23 d 5 2. At [Xe]6 s 24 f 145 d 106 p 5

Big Bang – Sheldon • Video

Valence Electrons (V. E. ) • Electrons in the atom’s outermost energy level • Determine the chemical properties of an element • V. E. are used in forming chemical bonds

Examples • Write the electron configuration and give the number of valence e -. Mg 2 valence e. Br 7 valence e. V 2 valence e-
![Exceptions 1 Cu not Ar4 s 23 d 9 but Ar4 s 13 Exceptions 1. Cu • not [Ar]4 s 23 d 9 but [Ar]4 s 13](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-40.jpg)
Exceptions 1. Cu • not [Ar]4 s 23 d 9 but [Ar]4 s 13 d 10 2. Ag • [Kr]5 s 14 d 10 3. Au • [Xe]6 s 14 f 145 d 10
![Exceptions 4 Cr Ar4 s 13 d 5 5 Mo Kr5 s 14 d Exceptions 4. Cr [Ar]4 s 13 d 5 5. Mo [Kr]5 s 14 d](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-41.jpg)
Exceptions 4. Cr [Ar]4 s 13 d 5 5. Mo [Kr]5 s 14 d 5

Ions • • Cations (+ ions) –remove e. Anions (- ions) - add e. O: 1 s 22 p 4 O 2 -: 1 s 22 p 6 Ne 2 O is isoelectronic with ____.

Examples Write the electron configuration for: • P 3 -: • 1 s 22 p 63 s 23 p 6 • Al 3+: • 1 s 22 p 6 • Ba 2+: • [Xe]
![Examples Pb Xe6 s 24 f 145 d 106 p 2 Examples • • • Pb: [Xe]6 s 24 f 145 d 106 p 2](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-44.jpg)
Examples • • • Pb: [Xe]6 s 24 f 145 d 106 p 2 Pb 2+: [Xe]6 s 24 f 145 d 10 Pb 4+: [Xe]4 f 145 d 10
![Transition Metals Fe Ar4 s 23 d 6 Fe 2 Ar3 Transition Metals • • • Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]3](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-45.jpg)
Transition Metals • • • Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]3 d 6 Fe 3+: [Ar]3 d 5
![Transition Metals Mn Ar4 s 23 d 5 Mn 2 Ar3 d Transition Metals • • Mn: [Ar]4 s 23 d 5 Mn 2+: [Ar]3 d](https://slidetodoc.com/presentation_image_h/c3b4aee571789c72d06a8fd02212ddfb/image-46.jpg)
Transition Metals • • Mn: [Ar]4 s 23 d 5 Mn 2+: [Ar]3 d 5 Mn 4+: [Ar]3 d 3 What is the highest possible charge for Mn? • +7

• Excited state: e- jumps to higher energy level • Ex: 1 s 22 p 63 p 6 • Ground state: normal econfiguration (lowest energy) • Ex: 1 s 22 p 63 s 23 p 1 • Blue Book: pg 358 # 37 -39

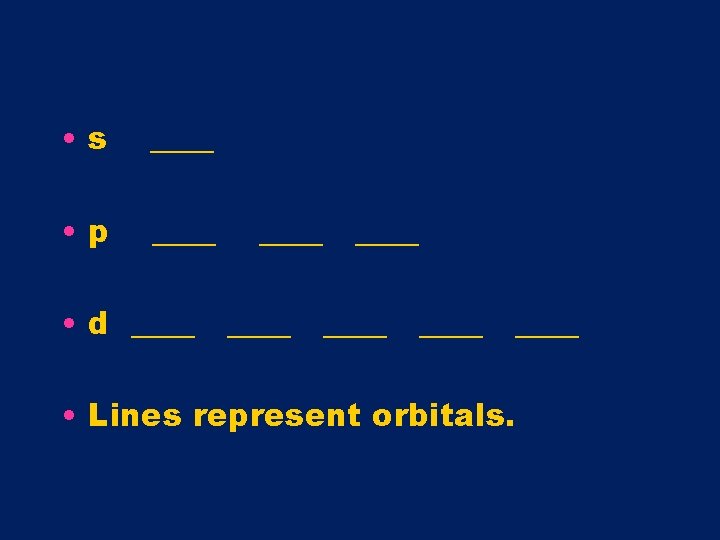
Orbital Diagrams • Use arrows to represent electrons • Use lines to represent orbitals • Every orbital can hold up to 2 e-

• s ____ • p ____ • d ____ ____ • Lines represent orbitals.


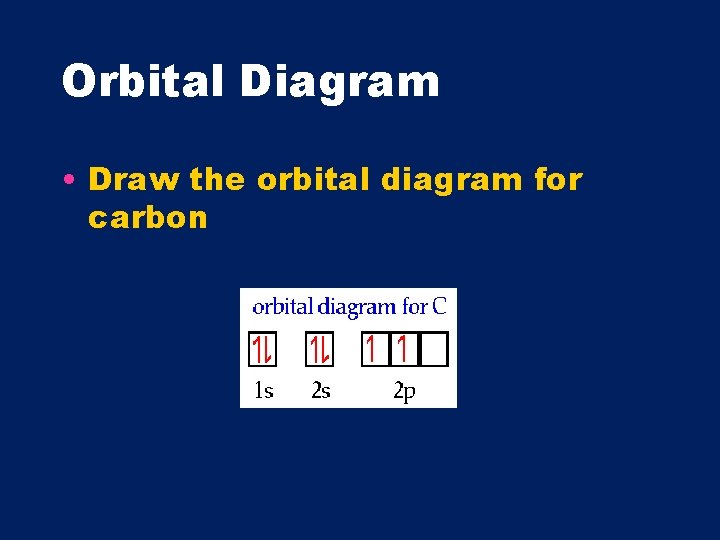
Orbital Diagram • Draw the orbital diagram for carbon

Hund’s Rule- atoms contain the maximum number of unpaired electrons

s p


 Are you sure you have a strategy
Are you sure you have a strategy Making inferences and drawing conclusions
Making inferences and drawing conclusions Quarter past an hour
Quarter past an hour Clock showing quarter past 7
Clock showing quarter past 7 I am sure you have heard something like
I am sure you have heard something like The triangle shirtwaist factory fire commonlit answer key
The triangle shirtwaist factory fire commonlit answer key How to avoid incremental plagiarism
How to avoid incremental plagiarism How did montresor know the house would be empty
How did montresor know the house would be empty Welcome 2nd quarter
Welcome 2nd quarter Welcome to quarter 2
Welcome to quarter 2 Welcome to quarter 3
Welcome to quarter 3 Xe6s24f145d10 element
Xe6s24f145d10 element I have chosen you and not rejected you
I have chosen you and not rejected you Epping forest community church
Epping forest community church Are you sure this is safe
Are you sure this is safe übring
übring A cube has 6 faces and 8 vertices
A cube has 6 faces and 8 vertices Wise men three clever are we
Wise men three clever are we Sometimes mistakes ... (make)
Sometimes mistakes ... (make) Make yourself welcome
Make yourself welcome Make the lie big, make it simple
Make the lie big, make it simple Steve angrisano go make a difference
Steve angrisano go make a difference Make the lie big make it simple
Make the lie big make it simple If she had studied harder
If she had studied harder Quote citation
Quote citation You must unlearn what you have learned
You must unlearn what you have learned What do you free time
What do you free time You have more potential than you think
You have more potential than you think May you be happy in the life you have chosen
May you be happy in the life you have chosen Where are you going where have you been true story
Where are you going where have you been true story Present perfect continuous conditional
Present perfect continuous conditional Hawk roosting annotations
Hawk roosting annotations Thank you for your attention do you have any questions
Thank you for your attention do you have any questions If i had 3 wishes they would be
If i had 3 wishes they would be Math quiz
Math quiz 49p11-843
49p11-843 Sure al alaq bedeutung
Sure al alaq bedeutung Possibility modal
Possibility modal Words with ture
Words with ture Sure design reviews
Sure design reviews Sure secure solutions
Sure secure solutions What is the overall purpose of management
What is the overall purpose of management Vertical line test
Vertical line test Christ is made the sure foundation lyrics
Christ is made the sure foundation lyrics Dr george papadatos
Dr george papadatos Sura at-tin
Sura at-tin 86 sure
86 sure Handheld bottle cap torque tester
Handheld bottle cap torque tester Inner defender examples
Inner defender examples Microsoft teamsrahman
Microsoft teamsrahman Najvaznije sure iz kurana
Najvaznije sure iz kurana High sure bets
High sure bets Toplam yağış eğrisi
Toplam yağış eğrisi Jede seele wird den tod kosten sure
Jede seele wird den tod kosten sure I sure hope nothing wacky and uncharacteristic happens
I sure hope nothing wacky and uncharacteristic happens Faith is being sure of what we hope for
Faith is being sure of what we hope for