Welcome to Physical Science Today you will learn

















- Slides: 22

Welcome to Physical Science! Today you will learn: 1. Why scientists use the scientific method. 2. The difference between theories and laws. 3. How matter is classified 4. The difference between physical and chemical properties.

What is Physical Science? I. Physical Science is the branch of science that focuses on non-living things. A. It often is broken down into two categories: Chemistry and Physics. B. In this class we will discuss the main concepts in chemistry and physics.

II. The scientific method A. The scientific method is used to solve a problem or explain an event. 1 -Make an observation 2 -Form a hypothesis 3 -Test the hypothesis 4 -Analyze the result 5 -Draw conclusion

Making Observations 1. An observation is information that you gather by your senses. (See, smell, hear, etc. )

Forming a Hypothesis 2. A hypothesis is a proposed answer to a question. This hasn’t been tested to see if it is true.

Experiments 3. Experiments are a step-by-step procedure for testing a hypothesis. a. Manipulated variable-the variable that you can adjust. (Ex: How fast you run in the experiment) b. Responding variable-the variable that changes in response to the manipulated variable. c. Controlled experiment-only one variable is changed at a time.

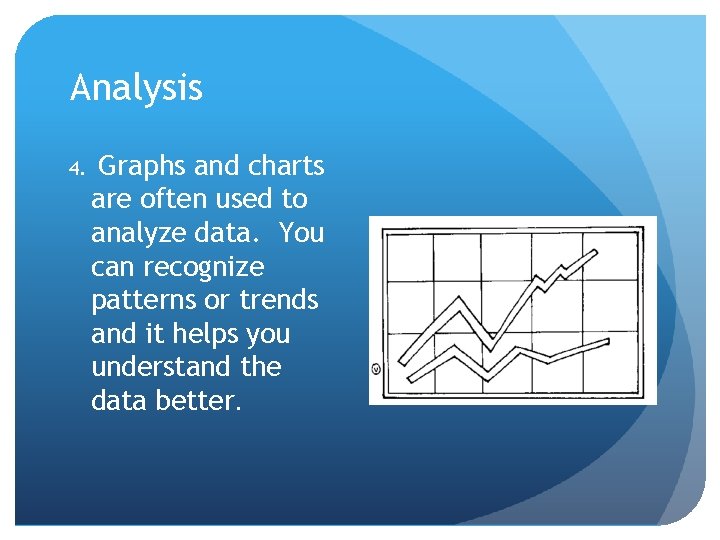
Analysis 4. Graphs and charts are often used to analyze data. You can recognize patterns or trends and it helps you understand the data better.

Conclusion E. The conclusion is your final thoughts regarding the experiment. -Was your hypothesis correct? -Do you need to redo the experiment?

Scientific Theory F. A scientific theory is a well tested explanation for an observation or experimental results. 1. 99. 9% truth 2. The process or event cannot be observed in its entirety. Ex: Big Bang Theory

Scientific Laws G. Scientific laws are statements that summarize a pattern found in nature. 1. 100% truth or fact. 2. Cannot be disproven. Ex: Newton’s Laws of Motion, Law of Universal Gravitation

II. Matter A. Matter is anything with mass that takes up space. 1. Matter is classified into different categories.

Classification of Matter B. Types of Matter 1. Pure Substances-matter that has the exact same composition throughout the entire substance. Ex: Table Salt (Na. Cl), Glucose (C 6 H 12 O 6) C. Elements have a fixed composition because it contains one type of atom. Ex: Aluminum Foil (Al) or Diamond (C)

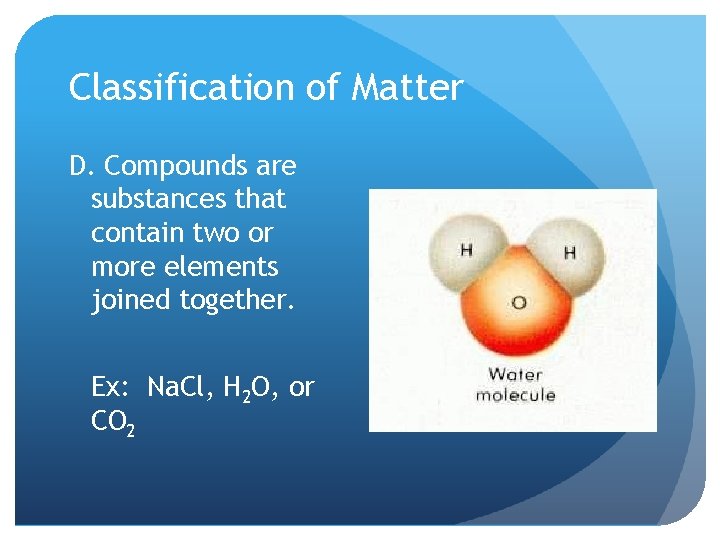
Classification of Matter D. Compounds are substances that contain two or more elements joined together. Ex: Na. Cl, H 2 O, or CO 2

Classification of Matter E. Mixtures contain different substances and its properties are different throughout the composition. 1. Heterogeneous Mixture-Different throughout Ex: Salsa, chicken noodle soup 2. Homogenous Mixture-Same throughout Ex: Kool-aid

III. Physical Properties A. A physical property is any characteristic of the material that can be observed or measured with changing the composition of the material.

Physical properties 1. Viscosity is a substance’s resistance to flow. Ex: Honey is more viscous than water.

Physical Properties 2. Conductivity- is a substance’s ability to allow heat or electricity to flow through it. Ex: Copper and water are very conductive! Styrofoam and wool are not conductive.

Physical Properties Malleability- is a substance’s ability to be hammered or stretched without breaking. Ex: metals are malleable, nonmetals are not

Physical Properties 4. Melting Point-the temperature at which a substance changes from a solid to a liquid. 32 °F or 0 °C for H 2 O 5. Boiling Point - the temperature at which a substance boils. 212°F or 100°C for H 2 O

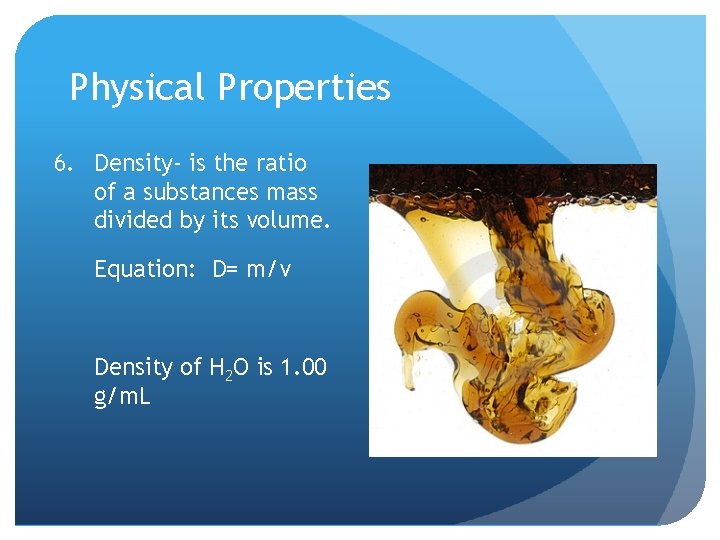
Physical Properties 6. Density- is the ratio of a substances mass divided by its volume. Equation: D= m/v Density of H 2 O is 1. 00 g/m. L

III. Chemical Properties A. A chemical property is any ability for a substance to change in chemical composition. B. There are two chemical properties: 1. Reactivity-it is able to go through a chemical reaction. Ex. Oxygen is very reactive and Nitrogen isn’t. 2. Flammability- it is able to burn in the presence of oxygen.

Recognizing Chemical Changes C. When something goes through a chemical change, it becomes a new substance. D. Evidence of chemical changes: 1. 2. Change in color- Rusted nail. Produces a gas-an Alka Seltzer tablet fizzes or bubbles when placed in water. 3. Forms a precipitate-sour milk curdles.